
The History of Cadmium Colors Begin with Medicine
Zinc oxide has long been used in medicine. One of the oldest references to its medicinal properties was found in an ancient Indian medical text, The Charaka Samhita, dating from 500 BCE. In the nineteenth century, apothecaries of Hanover, Germany, made zinc oxide by heating a naturally occurring form of zinc carbonate called calamine—a term referring to various zinc-based minerals.
Quality control of pharmaceuticals was entrusted to government-appointed physicians. In 1817, one such physician, Johann Roloff, became suspicious of a batch of zinc oxide that could be traced back to the factory of Karl Hermann. Roloff’s preliminary tests suggested that the samples contained arsenic, so Hermann—worried about the reputation of his business—investigated further. When in solution, samples of the batch turned a suspiciously yellow color with hydrogen sulfide and hence were believed to contain arsenic. The samples were tested for iron or lead contamination, as those were the typical culprits that caused the color change. No traces of either of those elements were found. It was also known at the time that arsenic turned yellow when exposed to sulfides to become another pigment, orpiment, or King’s Yellow—the synthetic form of arsenic sulfide.

The popular topical medicine calamine led to the discovery of cadmium sulfide.
Meanwhile, In 1817, Friedrich Stromeyer, Inspector-General of the apothecaries in Hanover and professor at Göttingen University, investigated some puzzling zinc carbonate samples that, upon heating, left behind a yellow oxide.
The intertwined roles of Roloff, Hermann, and Stromeyer—who corresponded and wrote about their findings—make it virtually impossible to give someone sole credit for identifying the new element.[1] When Stromeyer looked into the problem, he traced the yellow to brown discoloration to a substance he could not identify, which he concluded must be an unknown element. He separated the brown oxide and, by heating it with lampblack, produced a sample of a blue-grey metal, which he named cadmium, after the Latin root, cadmia. In alchemy, cadmia is an oxide of zinc that collects on the sides of furnaces where copper or brass was smelted and zinc sublime.
Origin of the Name Cadmium
The name cadmia is derived from the Greek καδμεια γη [kadmeia gè], which means cadmean earth or calamine, a zinc compound. Cadmean earth was first found near Thebes, founded about 1450 BCE by the Phoenician prince Καδμος [Kadmos, in Latin Cadmus], son of the Phoenician king Agenor, and brother of Ευρωπη [Eurōpè]. Καδμεια [Kadmeia] was also the name of the fortress of Thebes, named after its founder. The legend tells that Cadmus was the first to find zinc and to notice that it gave a golden hue to copper during smelting.
Meanwhile, two other Germans, Karl Meissner in Halle and Karl Karsten in Berlin were working on the same problem and announced their discovery of cadmium the following year. Stromeyer was the first to publish a complete account of his investigations into the properties of the new substance in September 1818, which he christened cadmium. Karsten proposed calling it melinium, which is the yellow color of quince. In the Annalen der Physik he edited, Ludwig Wilhelm Gilbert proposed the name of junonium from the planet Juno. Kluge and Staberoh, who came to the same conclusion that it contained a new metal, suggested the name klaprothium after the German chemist Martin Heinrich Klaproth, who died the year before. However, the name cadmium—initially proposed by Stromeyer—stuck. [2]

The rare mineral greenockite is composed of cadmium sulfide.
The Discovery of Cadmium Element
The discovery of cadmium came at a time in scientific research when many new elements were found. It was the first metal found in a compound, not an ore. Traces of it were soon found in zinc ores, but it was not until after the lapse of twenty years from the time of Stromeyer’s publication that an ore of cadmium was discovered. [3, 4] General Charles Murray Cathcart, Lord Greenock at the time, described a mineral found on his estate. The mineral was cadmium sulfide, analogous to zinc sphalerite or galena. The new ore was called greenockite. [8] Since then, it has been found in various localities; however, it is a scarce mineral. Strohmeyer found cadmium in many other zinc compounds, including several supposedly pure samples. He estimated that cadmium comprised between 0.1 and 1 percent pure zinc and zinc compounds. For commercial purposes, cadmium is obtained from zinc and lead ores.

Charles Cathcart, 2nd Earl Cathcart, styled Lord Greenock, was an amateur geologist who discovered the cadmium mineral greenockite.
The very property that led to the condemnation of zinc white and ultimately brought about its discovery is the yellow color, now most frequently used in artists’ paint.
Origin and History of Cadmium Pigments
When first introduced, there were few stable, bright pigments in the yellow to red range, with stable orange and bright red rare. The cadmium pigments eventually replaced compounds such as mercury sulfide (vermilion) with improved lightfastness.
Cadmium combines readily with sulfur to form cadmium sulfide (CdS)—a yellow solid. Cadmium sulfide was suggested as a pigment in 1819 by Stromeyer, but it was not commercially available until the 1840s due to the scarcity of metal required for its production. A range of yellow inorganic pigments based on cadmium was commercially introduced around the middle of the nineteenth century. By the 1840s, cadmium was being produced industrially, and a small amount of the yellow pigment became available to artists. [6] Winsor and Newton introduced cadmium yellow to Britain under the name ‘aurora yellow’ in the Great Exhibition of 1851, held at the Crystal Palace. [11] Cadmium sulfide was renowned as a bright yellow pigment with excellent lightfastness and stability properties, but it was also difficult to obtain and expensive.

Winsor & Newton introduced cadmium yellow under the name ‘aurora yellow’ in the Great Exhibition of 1851, held at the Crystal Palace.
First Occurrence of Cadmium Pigments in Art
It has since become a staple on artists’ color palettes called ‘cadmium yellow.’ Combining cadmium sulfide with zinc sulfide produces greenish-yellow or pale-yellow colors. Artists have embraced cadmium pigments since the middle of the nineteenth century.
Cadmium yellow, or cadmium sulfide (CdS), is synthesized one of two ways: either by a “dry” process, where cadmium metal or cadmium oxide/carbonate is calcined with sulfur at 300–500°C, producing hexagonal cadmium sulfide (h-CdS) or by a “wet” process, where cadmium sulfide is precipitated by reacting cadmium salt with a soluble sulfide, favoring the production of cubic cadmium sulfide (c-CdS). Other non-crystalline or amorphous phases can also be formed during either synthesis.
The abundance of colors from cadmium compounds is because they are semiconductors with a bandgap in the visible spectrum. Cadmium sulfide has a bandgap of 2.42 eV (512 nm) and, therefore, absorbs blue, indigo, and violet—the human eye perceives the spectral complement as yellow.
Cadmium red pigment is cadmium sulfoselenide (CdS, CdSe) produced by co-precipitating and calcining, at high temperatures, a mixture of cadmium sulfide and selenide sulfide in varied ratios, forming a partially crystalline structure with sometimes hexagonal or cubic forms. Cadmium pigments are the most durable yellow, orange, and red inorganic pigments commercially available. They have excellent chemical and heat stability and can be used in chemically aggressive environments and durable applications without color fade. Cadmium sulfoselenide pigments were developed in response to the need for stable shades of cadmium orange to red colors. Cadmium and selenide salts are co-precipitated and then heated to 300°C.
Cadmium sulfide selenides (cadmium sulfoselenides) were commercialized initially in 1910. The manufacturing process involved heating cadmium sulfide, sulfur, and selenium to about 600°C in an inert atmosphere. An improved process developed by the German company Bayer in 1919 involved precipitating the aqueous solutions of alkaline sulfides and selenides. The residue was yellow, and the red shade developed upon heating at 300°C.
Cadmium pigments are highly saturated with good permanence and tinting power. Cadmium yellow, orange, and red are familiar artists’ colors and were frequently employed as architectural paints since they can add long life, color saturation, and covering power to coatings. Their most important use is in the coloring of plastics and specialty paints, which must resist high processing or service temperatures. Cadmium pigments were used extensively to paint pipes or glass—for example, red traffic lights or the stars on the Moscow Kremlin.

The stars on top of the Kremlin towers contain cadmium and selenium glass.
Sources of Cadmium and Its Applications
Cadmium is a relatively rare element and is not found as a pure metal in nature. It mainly occurs in association with zinc, lead, and copper sulfide ores. Cadmium was produced commercially in the twentieth century as a by-product of the zinc refining industry. Annual production and consumption have been relatively flat over the past 20 years. To minimize the risk of exposure to cadmium, its applications have gradually been restricted to a limited number of professional and industrial uses, where appropriate measures are in place to minimize exposure. Of all the cadmium produced worldwide, about 25,600 metric tons (tonnes) annually (2019), only three percent of it goes toward pigment manufacturing. Twenty-three percent is used in nickel-cadmium battery production. A whopping 57% is used in the manufacture of jewelry.
Cadmium makes the jewelry coating shiny in jewelry, adding weight and mass to each piece. The jewelry manufacturers substituted cadmium, antimony, and barium due to restrictions on lead in consumer products. [13] The following changes were prompted by a 2010 investigation that found Asian manufacturers were using metal to make children’s jewelry. Many countries banned cadmium in children’s jewelry, and testing found cadmium virtually disappeared from jewelry by 2012. The regulations did not address cadmium in adult products. [14] Keep that jewelry away from children!
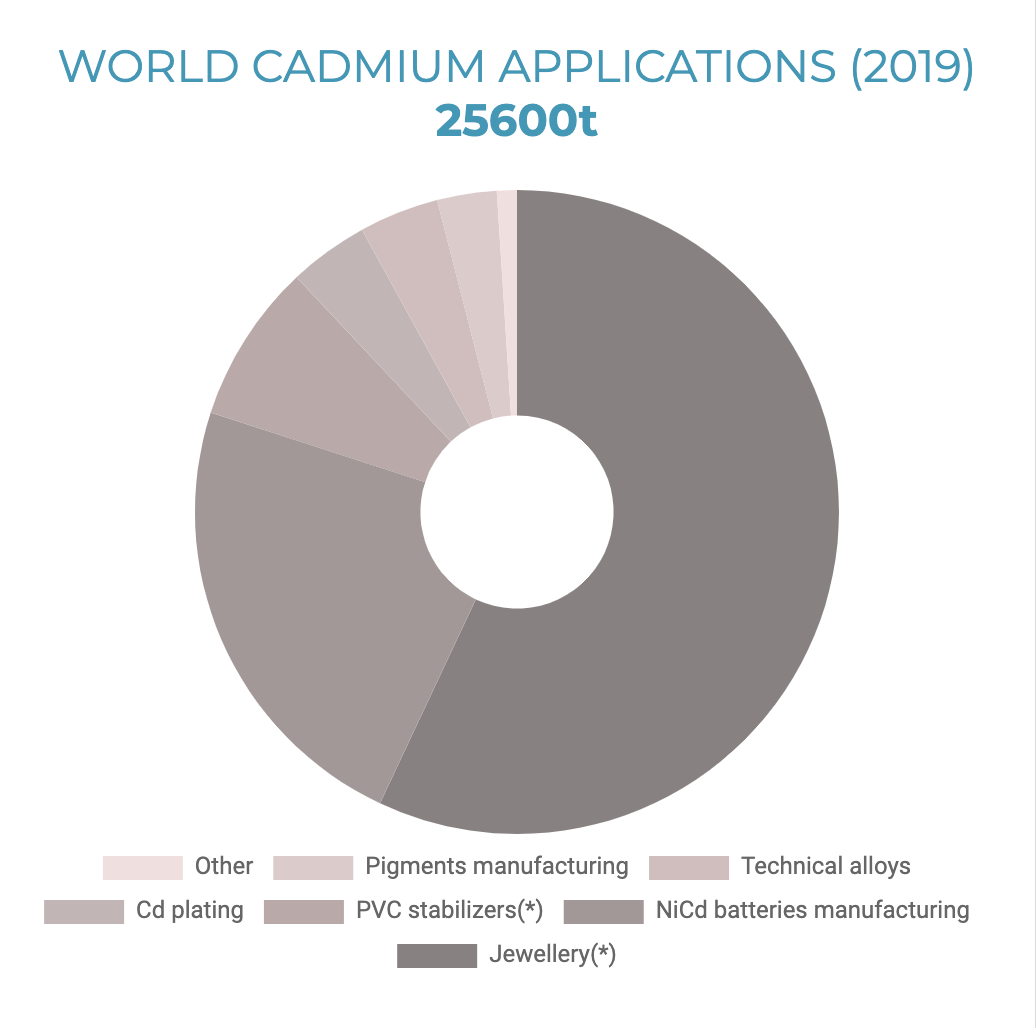
World Cadmium Applications 2019 (Source: International Cadmium Association)
* This application of cadmium is not permitted in the European Union.
The following are commonly used cadmium pigments in artists’ paints:
Light shades of cadmium yellow are cadmium zinc sulfide, typically a greenish-yellow solid solution of CdS and ZnS. Colour Index Pigment Yellow 35 (PY 35).
Deep shades of cadmium yellow are cadmium sulfide (CdS). Colour Index Pigment Yellow 37 (PY 37).
Cadmium orange is cadmium sulfoselenide, a solid solution of CdS and CdSe. Depending on the sulfur-to-selenide ratio, different Colour Index designations are assigned. For example, Colour Index Pigment Orange 20 (PO 20) or Colour Index Pigment Red 108 (PR 108).
Cadmium red is cadmium sulfoselenide. Colour Index Pigment Red 108 (PR 108).
Cadmium green is not a single pigment but a mixture of cadmium yellow (PY 35) and viridian (PG 18), giving a bright, pale green mixture. Some artist’s materials companies combine cadmium yellow (PY 37) and viridian (PG 18) (Holbein Artists’ Oil Colors) or phthalocyanine blue (PB 15:3) (Kremer Pigmente), or ultramarine blue (PB 29) (Langridge Artist Colors) instead to give a deeper green hue. There are even cadmium green hues that do not contain cadmium pigment but are complex mixtures of organic and inorganic pigments, such as cadmium green hue by Daniel Smith, which consists of nickel antimony titanium yellow rutile (PY 53), quinophthalone yellow (PY 138), and viridian (PG 18).
Uses of Cadmium Pigments
Most cadmium pigments are used in plastics and coatings. These pigments disperse well in most polymers and paint binders, giving high opacity and tinting strength. The pigments are insoluble in organic solvents, have good resistance to alkalis, and, in most cases, will remain lightfast for the application’s life. As a result, cadmium pigments have been used in a wide range of plastic products and coatings. Nowadays, their most significant application is in complex polymers processed at higher temperatures, requiring a cadmium pigment’s exceptional durability and technical performance. Their use is almost mandatory in many nylon, acrylonitrile butadiene styrene (ABS), polycarbonates, high-density polyethylene, silicone resins, and other modern thermoplastic polymers processed at high temperatures, which preclude the use of organic pigments and also most alternative inorganic pigments in the range of hues provided by cadmium. Cadmium-pigmented engineering polymers such as ABS are widely used in products such as telephones, gas pipes and fittings, electricity cables, beverage crates, and motor vehicle radiator fans.
Bright cadmium yellows, oranges, and reds are primary pigments for artists’ colors, where their permanence and opacity are the accepted standards against which other pigments are judged. Cadmium yellows and reds can have service temperatures well above 300 °C and are used in coatings for process chemical and steam pipes. They can also be incorporated into latex and acrylic coatings.
Permanence and Compatibility of Cadmium Pigments
Cadmium pigments are universally compatible and are recommended for high-performance applications where thermal stability, chemical stability, and weatherfastness are critical parameters.
According to the ASTM standard D4302 for oil and alkyd paints, cadmium yellow and red pigments are rated as Lightfastness Category I. While cadmium yellow and red pigments are considered lightfast on the artists’ palette, historical cadmium yellow pigments are known to degrade and alter color, either darkening or fading. Historical samples of pigments are a mixture of either h-CdS and c-CdS, combined phases of CdS, or hexagonal ZnxCd1-xS. Modern pigments are typically composed of only h-CdS or ZnxCd1-xS. [15]
The lightfastness or permanence of cadmium requires protection from a tendency to slowly form carbonate salts with exposure to air. Most paint vehicles accomplish this, but cadmium colors will fade in fresco or mural painting.
Oil Absorption and Grinding of Cadmium Colors
Cadmium red (cadmium sulfoselenide) absorbs a moderate amount of oil, about 20 grams of linseed oil per 100 grams of pigment, to make a paste. Cadmium yellow (cadmium zinc sulfide) absorbs a moderate amount of oil, about 21 grams of linseed oil per 100 grams of pigment, to make a paste.
The Real Story of Cadmium Pigment Toxicity
Animal studies have shown that cadmium pigments are potential carcinogens when inhaled. For this reason, paint manufacturers do not recommend spray applying cadmium paint. Cadmium sulfoselenide and cadmium sulfide are not very toxic (LD50 > 5000 mg/kg) when used as a pigment. They are not believed to be toxic by ingestion if they are of low solubility. For some time, pigment manufacturers have produced progressively lower solubility cadmium pigments to increase safety. Cadmium pigments today are highly insoluble compounds of calcined cadmium zinc sulfide and cadmium sulfoselenide. Solubility tests of cadmium compounds mimic conditions, such as pH, temperature, and agitation found in the stomach. However, other mechanisms at work in the body may not conclusively prove the results of solubility. Using low-solubility cadmium pigments diminishes their toxicity, but there are still reasons to treat paints made with cadmium pigments with care.
The level of cadmium solubility is tightly controlled to ensure compliance with Council of Europe Resolution AP (89) 1 Food Contact Limits; USA TCLP Waste Leaching Limits; Safety of Toys Directive 88/378/EEC (EN71: Part 3 relates to finished toy compliance). Cadmium pigments were registered as non-hazardous substances under REACH in 2013 and are regulated under Annex XVII of the REACH legislation (1907/2006).
For more information on handling pigments, please visit How to Safely Handle Art Materials and Pigments.
References
- Fontani, M., Costa, M. and Orna, M. V. The Lost Elements: The Periodic Table’s Shadow Side (Oxford Univ. Press, 2014).
- Cadmium. Elementymology and Elements Multidictionary. Online.
- Joy, C.A. “Cadmium and Its Uses.” Scientific American 21, 12, 181–182 (September 1869). doi:10.1038/scientificamerican09181869-181b
- Tarakina, N. and Verberck, B. A portrait of cadmium. Nature Chem 9, 96 (2017). https://doi.org/10.1038/nchem.2699.
- W. G. Huckle, G. F. Swigert, and S. E. Wiberley, “Cadmium Pigments. Structure and Composition”, Industrial & Engineering Chemistry Product Research and Development, 1966, 5 (4), 362–366.
- Allen, E.T. and Crenshaw, J.L. Amer. J. Sci., 34 (1912) 341.
- Stromeyer, F. Ann. Phil. (trans. from Annalen der Physik), 14 (1819) 269.
- Connell, A. “Chemical Examination of Greenockite or Sulphuret of Cadmium,” The Edinburgh New Philosophical Journal, (1840) 392.
- Technical notes on cadmium and cadmium pigments; London: Cadmium Association, 1978.
- Curtis, P.J. and Wright, R.B. J. Oil Col. Chem. Assn., 37 (1954) 26.
- Engel, B.L. “A Study of the Materials and Techniques Used by Vincent Van Gogh,” Ohio: Intermuseum Laboratory, Ohio, 1975.
- “The size of the stars on the towers of the Kremlin. Secrets of the Kremlin ruby stars.” IK-PTZ. Online.
- Pritchard, Justin. “U.S. consumer chief warns Asian firms on cadmium use.” MPR News, January 11, 2010, 1:56 PM. Online.
- “Tests show popular stores sold toxic jewelry made with cadmium.” Associated Press, WXYZ Detroit. Online.
- Marta Ghirardello, Sara Mosca, Javier Marti-Rujas, Luca Nardo, Aviva Burnstock, Austin Nevin, Maria Bondani, Lucia Toniolo, Gianluca Valentini, and Daniela Comelli. “Time-Resolved Photoluminescence Microscopy Combined with X-ray Analyses and Raman Spectroscopy Sheds Light on the Imperfect Synthesis of Historical Cadmium Pigments,” Analytical Chemistry 2018 90 (18), 10771–10779. DOI: 10.1021/acs.anarchism.8b01666
Pigment Names and Specifications
Cadmium Red Pigment
| Pigment Names | |||||||
| Common Names: | English: cadmium red French: cadmium rouge German: Kadmiumrot Italian: cadmio rosso Russian: Кадмий красный Spanish: cadmio rojo |
||||||
| Nomenclature: |
|
||||||
| Pigment Information | |
| Color: | Red |
| Pigment Classification: | Synthetic Inorganic |
| Colour Index: | Pigment Red 108 (77202) |
| Chemical Name: | Cadmium Sulfoselenide |
| Chemical Formula: | CdS, CdSe |
| CAS No.: | 58339-34-7 |
| ASTM Lightfastness | |
| Acrylic: | I |
| Oil: | I |
| Watercolor: | I |
| Physical Properties | |
| Particle Size (mean): | 0.9 microns |
| Specific Gravity: | 5.12 |
| Bulk Density: | 0.99 g/ml |
| pH: | 6.0 |
| Refractive Index: | 2.384 |
| Oil Absorption: | 20 grams oil / 100 grams pigment |
| Heat Stability: | 400 °C |
| Health and Safety |
WARNING! Contains Cadmium Compound. Classified by IARC as probably carcinogenic in humans. Avoid breathing dust, mists, or spray. Do not sand. Keep out of reach of children. Conforms to ASTM D-4236. California Proposition 65: Contains a chemical known to the State of California to cause cancer by means of inhalation. |
Cadmium Yellow Pigment
| Pigment Names | |||||||
| Common Names: | English: cadmium yellow French: cadmium jaune German: Kadmiumgelb Italian: cadmio giallo Russian: Кадмий желтыйсветлый Spanish: cadmio amarillo |
||||||
| Nomenclature: |
|
||||||
| Pigment Information | |
| Color: | Yellow |
| Pigment Classification: | Synthetic Inorganic |
| Colour Index: | Pigment Yellow 35 (77205) |
| Chemical Name: | Cadmium Zinc Sulfide |
| Chemical Formula: | CdS, ZnS |
| CAS No.: | 8048-07-05 |
| ASTM Lightfastness | |
| Acrylic: | I |
| Oil: | I |
| Watercolor: | I |
| Physical Properties | |
| Particle Size (mean): | 0.7 microns |
| Specific Gravity: | 4.71 |
| Bulk Density: | 0.91 g/ml |
| pH: | 5.7 |
| Refractive Index: | 2.384 |
| Oil Absorption: | 21.8 grams oil / 100 grams pigment |
| Heat Stability: | 400 °C |
| Health and Safety |
WARNING! Contains Cadmium Compound. Classified by IARC as probably carcinogenic in humans. Avoid breathing dust, mists, or spray. Do not sand. Keep out of reach of children. Conforms to ASTM D-4236. California Proposition 65: Contains a chemical known to the State of California to cause cancer through inhalation. |
For a detailed explanation of the terms in the table above, please visit Composition and Permanence.
Frequently Asked Questions
What color and texture is cadmium?
Cadmium pigments range in color from bright yellows to deep reds. They have a dense, opaque texture, providing excellent coverage and color saturation in paint formulations. The texture in paint depends on the medium used, contributing to vibrant and lasting hues in oil, acrylic, and watercolor applications.
What is the difference between cadmium and hues?
Cadmium pigments are inorganic compounds that provide vivid, durable colors. "Hues" are imitations made from organic pigments or mixtures designed to mimic the appearance of cadmium colors. While hues may closely replicate the visual aspects of cadmium colors at full strength, they often lack the same color fastness, covering power, and vibrancy of genuine cadmium pigments.
What is cadmium in acrylic paint?
Cadmium is a vibrant, highly opaque pigment in acrylic paint. Known for its excellent lightfastness and covering power, cadmium offers artists a range of warm colors, from bright yellows to deep reds. It is prized for its durability and the ability to maintain its hue even when mixed with other colors or exposed to light over time.
What shade is cadmium red?
Cadmium red is a deep, warm, slightly orange shade of red. This pigment is celebrated for its color intensity, excellent covering power, and resistance to fading. Cadmium red offers artists a reliable and vibrant hue that is consistent and long-lasting in various artistic mediums.
What is the color code (Colour index) for cadmium red, orange, and yellow?
The Colour Index International (CII) provides specific codes for pigments: Cadmium Red is often associated with codes like PR108 (Pigment Red 108), Cadmium Orange with PO20 (Pigment Orange 20), and Cadmium Yellow with PY37 (Pigment Yellow 37). These codes help identify the exact chemical composition and hue of cadmium pigments used in artistic and industrial applications.



















