The lead white found in artists’ oil paints is the modern form of the pigment, whereas the lead white in all paintings made before the 20th century contains the ‘traditional’ (stack process or old Dutch method) form of lead white. So what is the difference?
Lead white is the most important white pigment used in painting throughout history. It was known to the ancient Egyptians, Greeks, and Romans and was commonly used to prepare ointments, plasters, and cosmetics. It was first identified in literature as a pigment by Pliny, who mentions it, among other colors, as used by the ancients to paint ships.
Although Davy failed to find lead white in his examination of the pigments discovered in the excavations at Pompeii,[1] he believed that it was commonly used and that the ancient Romans prepared lead pigments of different hues between Pliny’s usta or minium and partially calcined lead white or massicot.[2] The ancient Romans attained considerable skill in the preparation of pigments, and the manufacture and sale of colors was a well-established branch of industry and commerce in the Roman empire. One of the shops discovered in the excavations at Pompeii had jars of pigments displayed in long rows, ready for sale to the artist and painter.
Lead white is lead carbonate hydroxide (basic lead carbonate) with the chemical formula 2PbCO3, Pb(OH)2. It has been made since antiquity using variations of the process known as the ‘stack’ or ‘Dutch’ process, whereby lead is exposed to acetic acid vapors in the presence of moisture and carbon dioxide; the latter is generally provided by fermenting matter (horse manure, waste grape skins, tanbark), which also provides a constant source of heat. In this process, the air supplies the oxygen, while the fermenting matter produces carbon dioxide and moisture. The acetic acid, in the form of vinegar, converts the lead, forming basic or tribasic lead acetate, which is afterward decomposed by the carbon dioxide to form basic lead carbonate.
History of Lead White Manufacture until the Twelfth Century
The method of preparing lead white pigment, common in Europe until the twelfth century, was probably the same as that of ancient Grecian and Roman painters. Pliny mentions the use of a native ceruse (Middle English, from Middle French céruse, from Latin cerussa) found on the lands of Theodotus at Smyrna. “At the present day,” he continues, “all ceruse is prepared from lead and vinegar.” [3] The native ceruse suggests Ajasson, one of Pliny’s commentators, was native lead carbonate or cerussite. Ceruse, lead white, is prepared in much the same manner as described by Pliny as it was throughout history until the beginning of the twentieth century. Pliny provides the following detailed account of its preparation:
“It is made from very fine shavings of lead, placed over a vessel filled with the strongest vinegar; by which means the shavings become dissolved. That which falls into the vinegar is first dried, and then pounded and sifted, after which it is again mixed with vinegar, and is then divided into tablets and dried in the sun, during summer. It is also made in another way: the lead is thrown into jars filled with vinegar, which are kept closed for ten days; the sort of mold that forms upon the surface is then scraped off, and the lead is again put into the vinegar until the whole of the metal is consumed. The part that has been scraped off is triturated and sifted...” [4]
One of the few surviving early medieval Latin treatises, De coloribus et artibus Romanorum, attributed to Eraclius, written between the seventh and twelfth centuries, constitutes three books about Roman techniques of the applied and fine arts. Eraclius describes many subjects, such as how to prepare drying oils, make lead white, prepare wood for painting, etc. He says: “If you wish to make the white which is called ceruse, take lead plates and put them into a new jar, and so fill the jar with very strong vinegar and cover it up, and set it in some warm place, and leave it so for a month; then open the jar and put what you find adhering to the strips of lead into another jar, and place it upon the fire, and keep stirring up the color until it becomes as white as snow.” [5] This description is similar to those given by Theophrastus, Vitruvius, Pliny, and Dioscorides but would seem to result in lead acetate and not lead white or at least a mixture of the two substances. It is interesting to note that Eraclius, in another place, recommends using decomposing dung to furnish heat. He describes a process for making a green pigment: “Mix vinegar with strong honey, and then cover up the vase itself in very hot dung, and so take it out after twelve days have elapsed.” [6] This recipe occurs in the first book of Eraclius, which is acknowledged to be the earliest portion of the manuscript and is thought by many authorities to have been written before the tenth century.
In the tenth century, the author of the Mappae Clavicula describes making lead white: “Cast lead into plates or sheets, then suspend over strong vinegar. After it is corroded, scrape it off and wash well.” Another proposed method directs that lead cast in plates be placed in a new pot partly filled with vinegar; the pot is then covered closely, put in a warm place, and left undisturbed for a month. Upon opening the pot, the corroded lead should be removed and thoroughly washed “when it will become as white as snow.” [7]
Theophilus, a monk living in the twelfth century, gave instructions on how to make lead white: “If you are going to make ceruse, thin out some lead plates and lay them together, dry, in a wooden chest in the same way as the copper above. Then pour hot vinegar or urine to cover them. After a month pry off the lid and remove whatever white there is and replace [the plates] as before.”
After describing the method in which the lead plates should be prepared and placed in the wooden chest, Theophilus refers to the preceding recipes for directions for further treatment. In the recipe for making “salt-green,” which is a pigment prepared from copper plates, Theophilus gives essential directions for preparing the wooden chest: “Get some thin twigs and place them in the chest mentioned above in such a way that two-thirds of the cavity are beneath [the twigs] and the other third is above them. Then lay them [the plates] next to each on the twigs and cover them carefully with another piece of wood, fitted for this purpose, so that no vapors can escape. Next, in the corner of this piece of wood, drill a hole through which you can pour heated vinegar or hot urine until a third of [the chest] is filled; then block up the hole. Put this chest in a place where you can pile dung all over it.” [8] It appears, therefore, that the direction to bury the chest in dung is omitted simply to save a tedious repetition. Assuming this idea, we have in this description the recommendation of using manure as a source of heat and possibly a source of carbon dioxide necessary for producing lead white. There can be no question of using dung for similar purposes to make green copper pigments, as both Eraclius and Theophilus recommend it.
Centuries later, Didron found a manuscript in a convent at Mount Athos in Greece, portions of which the monks claimed to be unquestionably of Byzantine origin and copied as early as the tenth or the eleventh century. The manuscript received additions from time to time, and copies were frequently made from it for distribution. The following directions are found in this manuscript under the title, “How to Make Ceruse: Take lead cut into thin pieces, and suspend these pieces in a pot filled with vinegar; close tightly this pot, and bury it in fresh dung in a warm place. At the end of ten or fifteen days take up the pot and throw the lead upon a stone and grind it; put the product in a large vase and dry it, and you will have a good ceruse.” [9]
The descriptions of the methods used to make lead white in these manuscripts form a link between the methods described by the Roman and Greek authors and those in practice until the twelfth century. While in general, they resemble the methods recommended by Theophrastus, Vitruvius, and Pliny, inferring that the differences are only such as would naturally occur in their transmission by copying through ten centuries, yet Theophilus and Eraclius both imply that dung was used as a means of producing the necessary heat. The absence of any clue to provisions for supplying carbon dioxide required to produce lead white can be disregarded because the vinegar used in those times was probably largely contaminated with substances produced by their decomposition of carbon dioxide in sufficient quantities. There can be no doubt about respecting the product of the methods described by Theophilus and Eraclius.
Lead White Manufacturing from the Thirteenth to the Nineteenth Centuries
Among the manuscripts collected by Jehan Le Begue is one by Petrus de Sancto Audemaro, who lived in the north of France late in the thirteenth or early in the fourteenth century. Some of the formulas of this author are considered to be much older than his time, among which is the following: “White and green colors, without salt, are made and tempered as follows: pour strong vinegar into a vase and place twigs across it inside the vase, and then place strips of lead, and other strips of copper or brass, suspended in the air by means of twigs, so as not to touch the vinegar or each other; then close the vase very carefully and lute it with clay or cement or wax, so that there may not be the least hole through which the vinegar may exhale. Then cover it with horse-dung, and after thirty days, on account of the acidity of the vinegar or wine (for the wine, on account of the heat of the dung, will become vinegar), the copper or brass will be found to be turned green, and the lead white. Take the lead white, dry it, grind it, temper it with wine to paint on parchment, and mix it with oil to paint on wood.” [10] The vinegar recommended here is probably a mixture of wine lees (deposits of residual yeast and other substances that settle to the bottom of a vat of wine after fermentation) and wine or vinegar in which fermentation had begun but was incomplete because, as Audemaro says, “The wine, on account of the heat of the dung, will become vinegar.” Audemaro also instructs that the pots containing the lead and vinegar be buried in beds of dung, and in describing the manufacture of salt-green, he recommends that the operation be conducted in a horse stable. This author also suggests that grape pomace (the solid remains of grapes after pressing for juice, containing the skins, pulp, seeds, and stems of the grape) be used as a source of fermentation instead of horse manure, saying it will produce the same result.
A manuscript preserved in the library of the convent of Saint Salvatore, in Bologna and entitled Segretti per Colori is a collection of recipes for making and preparing colors for painting, dyeing, etc. The name of the author is unknown, but the date of the manuscript is considered to be about the middle of the fifteenth century. The compiler describes the manufacture of lead white as follows: “Take leaden plates, and suspend them over the vapor of vinegar in a vase, which, after being luted, must be placed in dung for two months; then scrape away the matter that you will find upon the plates, which is the lead white. Do this until the plates are consumed.” The author of this treatise adds the following instructions for purifying lead white: “Take ceruse, put it in a clean jar, which should be placed over the fire, stir the ceruse continually with a stick, and it will become white.” [11]
Pierre Le Brun wrote a painter who probably resided in Paris a manuscript in 1635, now in the Public Library in Brussels. Le Brun says that lead white is made “by putting vine branches in pots, pouring vinegar over them, fixing sheets of lead on the top, and fastening them up air-tight.” He mentions, in a list of white pigments, ceruse, blanc de Venise, and blanc de plomb, as if they were different substances.[12] Writers of his time generally refer to Venice lead white as being the best, and it is probable that lead white, which was then manufactured in Holland and perhaps in England, was adulterated and given different names, each indicating a certain degree of impurity.
The manufacture of lead white is described in a large number of the sixteenth and early seventeenth-century manuscripts, the earliest of which, MS. Sloane 122, includes a detailed explanation of the way in which a barrel should be prepared with two gallons of vinegar at the bottom and strips of lead suspended inside above the vinegar. After the barrel has been made as air-tight as possible, it should be left for eight weeks, when it can be opened up, and the white pigment knocked off the lead strips.[13] In discussing the methods of lead white in medieval times, Thompson points out that this class of medieval recipes for making lead white is different from the type that closely resembles the stack process.[14] In this class, the lead is suspended above vinegar in a pot, and the pot is then sealed up tightly and buried in hot dung. If the pot is sealed, it is hard to understand how the carbon dioxide can reach the lead and cause the reaction resulting in lead carbonate. Presumably, these methods only make lead acetate, which is the same objection to the methods described by writers, such as Pliny, before the twelfth century.
It is possible that this was actually the case; lead acetate was formed in these methods and not lead white because in these recipes’ products were regularly subjected to a special after-treatment not specified for lead white made by other methods. It was roasted gently in the open air. It is possible this roasting might, under some conditions, produce lead carbonate from the acetate. If this explanation is accepted, then we come to realize that there were two types of lead white in use since antiquity through medieval times: one much the same as stack process lead white, and another somewhat different. The differences may not have been that noticeable.
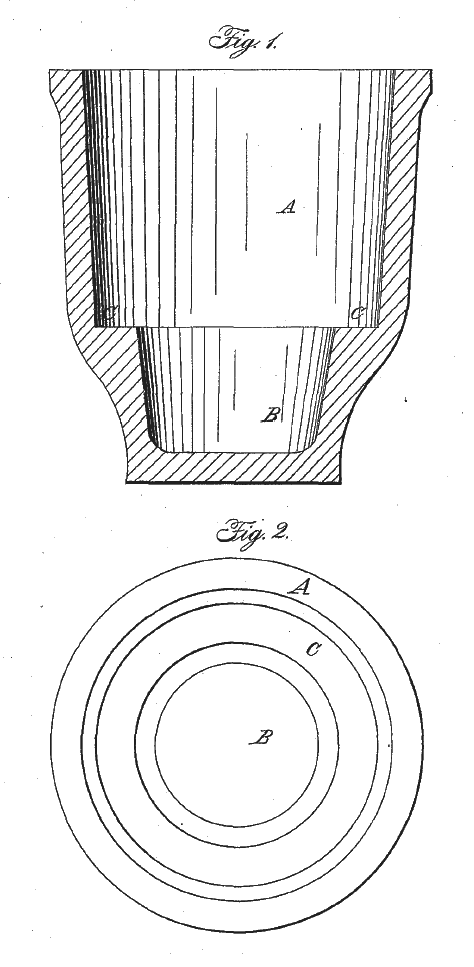
Corroding pot used in the stack process
Image from U.S. Patent No. 21,018, November 21, 1865
Lead white was manufactured on a large scale in England in the seventeenth century, and patents were issued for making white and red lead. Vernatti provided the Royal Society with a written account of a factory in the second half of the seventeenth century. Lead was cast in plates measuring one yard long and six inches wide, each being sufficiently thin so that it could be rolled loosely. Pots were prepared with a quantity of vinegar at the bottom and a bar across so that the coil of lead, which was placed inside, would be held above the vinegar. A plate of lead was placed over each pot to form a lid. A square bed of dung was prepared, and 400 pots were set in it in rows and covered with boards, which allowed another layer of pots to be arranged over it. Four layers were stacked and covered, and the entire stack of 1,600 pots containing lead coils and the cover was left standing for three weeks, after which time the lead covers and coils were removed and beaten to dislodge the white flakes. When the flakes had been separated from the remaining metal, they were ground with water between millstones. The pigment was then molded into small pieces and exposed to the sun to dry.[15] Later accounts during the eighteenth and nineteenth centuries are very similar to this detailed description of what was later called the stack process.
Very little alteration was made to the process in the eighteenth century, except for a patent, number 1581, taken out in 1787, that specified the use of tanner’s bark instead of layers of dung because it would produce a more consistent temperature within the stack and not emit sulfur-bearing gases that were considered to be the cause of discoloration observed in some of the product.
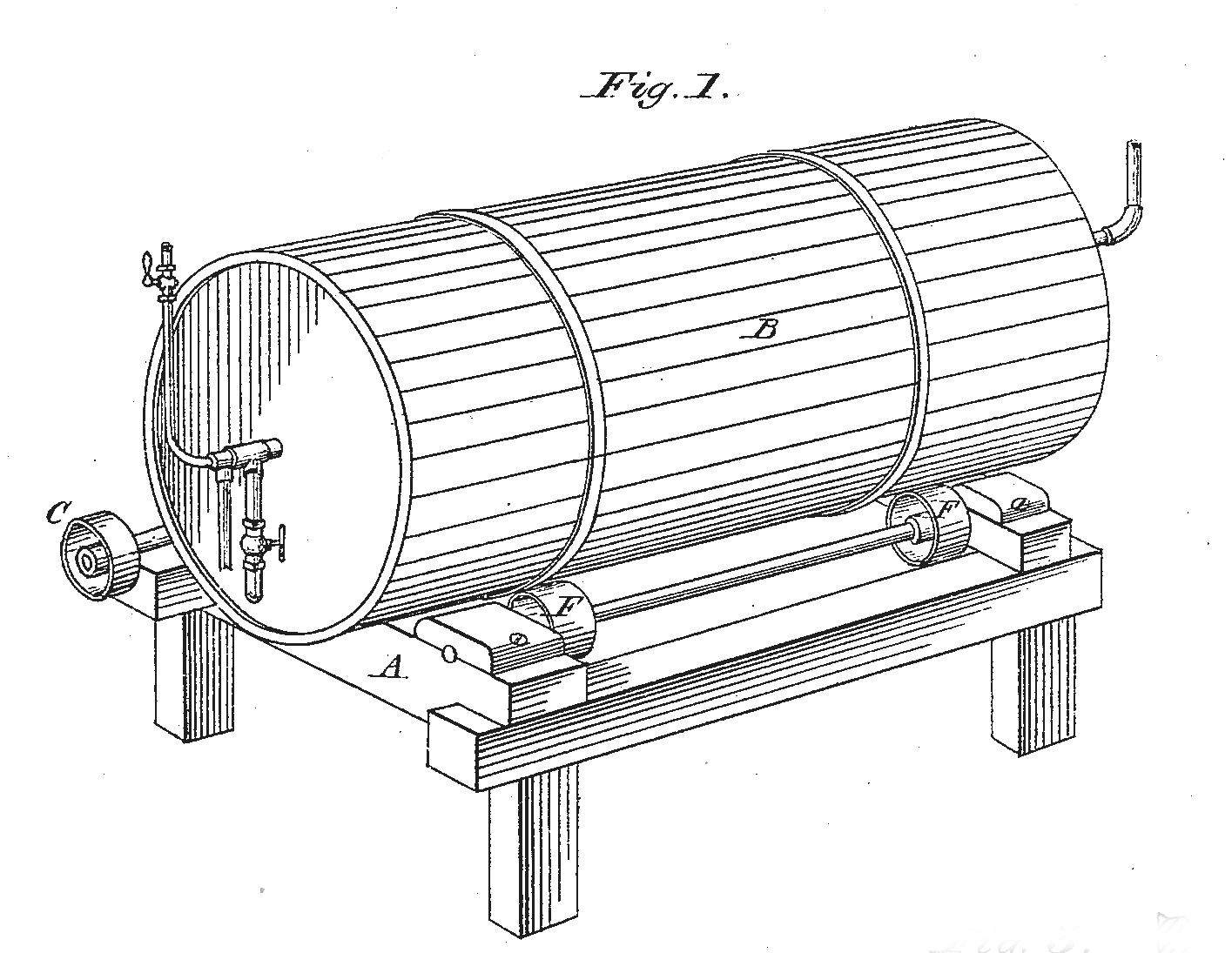
Corroding drum used in the Carter Process Image from U.S. Patent No. 148,862, March 24, 1874
Modern Manufacturing Processes of Lead White
The French process consists of subjecting a solution of basic lead acetate to a current of carbon dioxide gas, which forms a precipitate of lead white. The problem is that the French process tended to produce lead carbonate with a smaller proportion of lead hydroxide.
In the latter half of the nineteenth century, newer methods of manufacturing lead white were developed. Levi Carter, a freighter and cattleman in Omaha, Nebraska, experimented with a new process whereby the lead was reduced to small particles and then corroded. Typified by the Carter process (sometimes called the “quick process”), these methods consist of tumbling granular lead in a rotating drum into which acetic acid is sprayed along with a current of hot carbon dioxide and air. This method is said to produce an exceptionally uniform texture to the product.
Stack shed housed the corroding pots and horse manure or tan bark
Image from the U.S. Patent No. 252,823, January 24, 1882
Ernst Boehne of St. Louis patented a process for reducing lead into granulate form to reduce the time needed to corrode lead and make lead white (Patent No. 142,199, dated August 26, 1873). David Tuttle and James McCreary of Baltimore developed another method of granulating lead and then making lead white in a drum (Patent No. 148,862, dated March 24, 1874). John Stewart MacArthur of Glasgow, Scotland, was granted a United States patent (Patent No. 548,566, dated October 22, 1895) for a process of making lead white by heating litharge (lead oxide) in an alkaline solution.
A new development in the manufacture of lead white that represented a significant departure from previous methods was first described in a patent issued to Johann Kessler, a German immigrant living in Milwaukee, dated January 15, 1884 (United States Patent No. 292,119) and to Turner Bottome of Hoosick, New York (U.S. Patent No. 414,935, dated November 12, 1889). The patents described a method of making lead white using an electric current or electrolysis. David Kyte of Indianapolis was awarded Patent No. 459,946 on July 5, 1890, for his improvements to the electrolysis process. On August 11, 1893, Arthur Browne, Cambridge, Massachusetts, was assigned a patent, Patent No. 496,109, for his method of manufacturing lead white by electrolysis. Carl Luckow of Cologne-Deutz, Germany, patented an improved method of producing lead white by electrolysis first in England, Patent No. 14,801, dated August 6, 1895, and later in the United States, Patent No. 627,002, issued on June 13, 1899. The method consisted of placing the lead anode in an electrolyte aqueous solution of sodium, potassium, or ammonium chloride mixed with sodium, potassium, or ammonium carbonate.
Erratic Results of Early Manufacturing Methods
There was considerable variation between the amounts of lead white obtained from different pots; even pots set next to each other. Some yielded thick, good flakes, others were few and thin, and others were none. Sometimes the pots when removed from the stack were dry and sometimes the flakes on the lead coils proved best, while other pots still contained vinegar. In Vernatti’s description of the lead white manufacturing process, he observed that ‘the plates that cover the pots, yield better and thicker flakes, than do the rolls within and the outsides of the plates.’ He also noted that the outside of the plates had more lead white than the inside nearest the coils.
Importance of Lead White Pigment in Painting
The density of lead white and its opacity is very important to painters. If lead white were not dense and opaque, it would be necessary to put it on thickly where, since it is both dense and opaque, a thin coat of it will serve. The power that it gives of making strokes of light as incisive as strokes of dark is fundamental to the technique of medieval book and panel painting.
Modern Reproduction of Stack Process Lead White
At Natural Pigments, we wanted not only to understand the resulting product of the stack process but also the potential benefits that it could provide artists today, so we set out to reproduce the stack process. We built a ‘stack’ to reconstruct accurately the method of making lead white typified by the Dutch lead white industry of the sixteenth century. This method involved corroding lead strips coiled into rolls placed in earthenware pots on stacks of horse manure. The pictures below show the major stages of building the stack or what was called the “blue bed.”
 |
This shed was specially built to house the stack and control the environment in which the lead corrodes. |
 |
Strips were cut out of lead sheets that were degreased, cleaned, and rolled into spiral coils before loading into earthenware pots. |
 |
Lead coils were placed into the pots. Some pots were covered as part of our attempt to understand the corrosion process described in literature. |
 |
Fresh horse manure collected from local stables was mixed with bark and placed in layers inside the shed. |
 |
The corroding pots were transferred from the staging area to the shed and embedded in the horse manure. |
 |
Nearly half a ton of lead in pots was deposited over the three-foot-high bed of horse manure. |
 |
Stack pots after two weeks of corroding. |
 |
A close view of the coils shows the extent of corrosion on the lead after only two weeks, yet unevenly spread across the lead sheet. |
 |
Extensive corrosion on the lead coils just before tearing down the stack. |
 |
Notice discoloration on the upper parts of the lead rolls. |
 |
Genuine flake white as it drops from the rolls. |
 |
|
| Cross-section of modern lead white in linseed oil. | |
 |
|
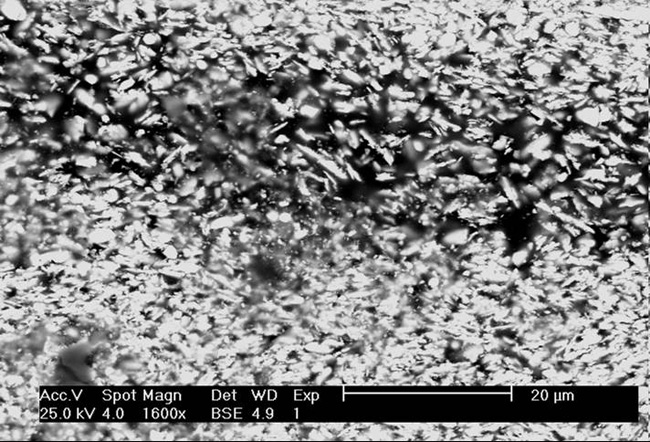
| Scanning electron microphotograph of modern lead white pigment. Images taken by Katrien Keune, FOM-AMOLF. |
|
 |
|
| Cross-section of lead white paint from a white highlight in Susanna, Rembrandt van Rijn (1636), Mauritshuis inv. no. 147. The cross-sections were made by Petria Noble, Conservation Department, Royal Picture Gallery Mauritshuis. Images taken by Annelies van Loon, FOM-AMOLF. |
|
 |
|
| Scanning electron microphotograph of stack process lead white pigment, modern reproduction. Images taken by Katrien Keune, FOM-AMOLF. |
|
| The above four images are posted here by permission of the authors, Leslie Carlyle and Maartjee Witlox, from “Historically Accurate Reconstructions of Artists’ Oil Painting Materials”, Tate Papers No.7. | |
A shed was specially built to house the stack and control the environment in which the lead corrodes. Redwood bark was used to improve drainage and aerate the fresh horse manure. Several tons of horse manure was collected from local stables, mixed with redwood bark, and placed inside the shed.
Strips were cut out of lead sheets that were degreased, cleaned, and rolled into spiral coils before loading into earthenware pots. Coils of lead sheets were more common in sixteenth-century Dutch lead white works than were lead buckles later used in English and American lead works. The buckles were often used together with lead sheets, especially in eighteenth-century England and afterward in the United States, just as tanbark replaced stable litter in many of these lead works.
Specially made earthenware pots were first arranged and then filled about one-third with vinegar. The differently colored pots indicate different amounts of lead or lead that were processed differently. We next placed the rolled lead coils into the pots. Some pots were covered as part of our attempt to understand the corrosion process described in literature.
Fresh horse manure collected from local stables was mixed with bark and placed in layers inside the shed.
The corroding pots were transferred from the staging area to the shed and embedded in the horse manure.
Nearly half a ton of lead in pots was deposited over the three-foot-high bed of horse manure. We anticipated that it would require about twelve weeks for the lead to completely corrode and form lead white.
We placed wireless sensors in the stack shed to monitor the temperature and moisture. If we had more funds for this project, we would have also liked to record the carbon dioxide levels in the stack. We recorded this data daily during the progress of the stack and took photographs at semi-monthly intervals.
In addition to this article, we reviewed many of the historical variations found in literature and that we have attempted to simulate in our stack.
Week Two
The stack shed is located in a remote area where the outside temperature varied from 5º to 25º C during the season in which it was started. The temperature continued to fall as the winter approached.
After two weeks of corroding, we opened the shed and immediately encountered the strong odor of vinegar inside the stack shed, but not as sharp as it was initially after building the blue bed. The temperature within the compost heap continued to increase, but the temperature inside the shed fluctuated with outside temperatures. The relative humidity inside the shed was nearly 100%. Controlling the compost stack temperature is critical to maintaining conditions suitable for lead corrosion. We developed an innovative method for maintaining the temperature inside the shed.
A close view of the coils shows the extent of corrosion on the lead after only two weeks, yet unevenly spread across the lead sheet. This was a problem often discussed in literature but without a solution.
Final Weeks Before Stack Tear Down
Although the stack shed was located in California, we had an unusual snowfall in February. We decided to wait until the snow cleared before tearing down the stack.
There was much corrosion observed on the rolls of lead, but because of the outside low temperatures during the winter, the overall inside stack temperature was not as high as we hoped, so we expected the yield to be lower than originally planned.
From a review of literature on the subject, it appears that lower stack temperatures result in a somewhat different particle structure, forming more compact rosettes. We hope to obtain scanning electron microscope photographs of the lead white particles to verify the particle structure.
Upon closer examination of some of the lead coils, we noticed discoloration of the flake white. We observed green, yellow, and rust-colored discoloration on parts of some rolls.
We readily could see why the white corrosion was called flake white. Large flakes of white dropped from the rolls as they were removed from the pots. The picture clearly shows the appearance of flake white; friable, flat pieces of white lead. At first, it is somewhat disconcerting how discolored some of the flakes appear, but this and other impurities are washed away when we began to levigate the white flakes.
Lead White Pigment for Artists’ Use
Rublev Colours® Lead White is made by a modern process yielding a finely divided basic lead carbonate powder of high purity. Rublev Colours Stack Process Lead White is made according to the stack process typified by the methods used by the sixteenth-century Dutch. The difference between lead white made according to modern methods and that made by the stack process is the size and shape of the pigment particles. The stack process yields a pigment with larger particles that vary more in size and shape. This difference affects the oil absorption ratio of the pigment, its opacity, and, most significantly, its consistency in oil paint. Both forms of basic lead carbonate usually contain about 70 percent of lead carbonate and 30 percent lead hydroxide.
Flake white is a term used to describe lead white or basic lead carbonate. The term originated with the stack process because the corroded lead white literally flakes off the metallic lead strips or buckles used in the process. The use of the term “Flake white” today by artists’ paint manufacturers is somewhat misleading because they do not make lead white paint with pigment made according to the stack process.
Analysis of Stack Process Lead White, Modern Reproduction
We received an independent laboratory analysis from Rutgers University of the various lead white pigments offered by Natural Pigments in the Rublev Colours line of dry pigments.
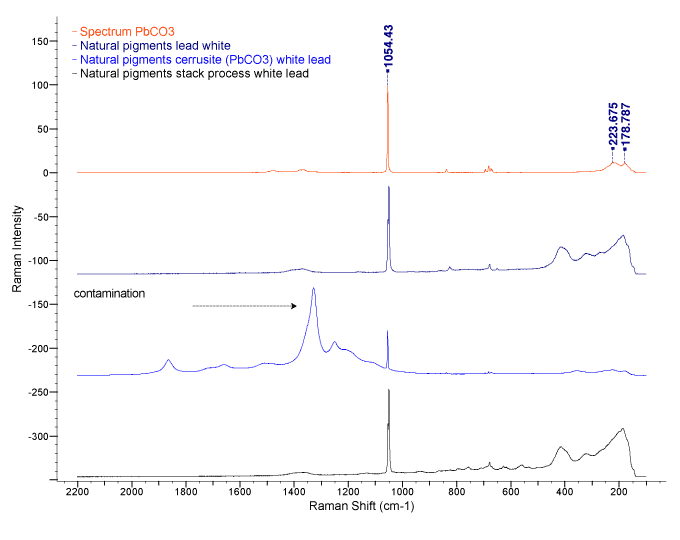
Raman spectroscopy was used to analyze the samples of lead white. Raman spectroscopy is commonly used in chemistry to characterize materials since the vibration information from laser excitation is very specific for the chemical bonds in molecules. It, therefore, provides a fingerprint by which the molecule can be identified.
The Raman spectra of the Rublev Colours Stack Process White Lead (black line) show that it is very pure basic lead(II) carbonate (2PbCO3.Pb(OH)2). This easily can be seen when comparing the spectra for the modern process lead white (dark blue line) to the stack process lead white.
The spectra indicated by the red line is a sample of pure lead carbonate (PbCO3) from Spectrum Laboratories. Basic lead carbonate or lead white is combined with an amount of lead hydroxide (between 20–40%), which is an important constituent of the pigment for artists’ use, giving it greater opacity and body in oil.
Analysis of different lead white by an independent laboratory at Rutgers University.
The results of the analysis are very interesting since the process used to make stack process lead white is an ancient method when compared to the methods used to make the modern lead white. We expected some contamination in the resulting product, such as that seen in the spectra for cerussite, which is predictable since this is an ore associated with accessory minerals found in nature. However, we were completely surprised to learn of its high purity.
This analysis leads us to the conclusion that the particle size and morphology (crystal structure and particle shape) of stack process lead white is the critical factor that makes this pigment useful as an artist’s color and superior in its handling characteristics to modern lead white.
The particle size and shape (morphology) of lead white pigment change the paint’s properties and its behavior when brushed. This is clearly demonstrated in the video clip of two lead white oil paint samples. The paint on the right is modern lead white, while the pile of paint on the left is made with stack process lead white. When brushing thixotropic paint, it flows; when stopped, it holds its shape. The particle size and shape of traditional pigments often provide thixotropy.
Modern lead white contains uniform and very fine pigment particles, typically a median particle size of 3 microns. Stack process lead white, on the other hand, typically contains larger particle-sized pigments in a variety of sizes and shapes. The pigment morphology and particle size distribution create the difference in paint behavior.
Resources
All of the pigments described in this article are available from Natural Pigments:
Stack process flake white oil paint made according to the method as described in this article is availalble from Natural Pigments:
Further Reading
For more information on stack process flake white, read this article, Notes About Stack Process Lead White, which discusses differences between modern lead white and our production of stack process flake white pigment. Another article, Variations of Stack Flake White, examines the properties of larger particle size stack process flake white have in oil paint.
Confused about the difference between flake white and Cremnitz white? The article Flake White and Cremnitz White explains the origins of these names and resolves the confusion.
For a complete description of white pigments used in artists' paints, please read the article titled White Pigments.
Notes
1. This appears to be confirmed by the comprehensive analyses of pigments conducted by the Italian chemist Selim Augusti in Pompeii in 1967. He detected a range of white pigments consisting of mostly chalk, but no lead white. That lead white was not detected in Pompeii is not at all surprising, considering that it appears to be well known to the ancient Romans the pigment could not be used in wall painting successfully. Pliny mentions lead white as being among the colors that are not to be used in wet stucco. Pliny writes, “Which colors do not admit being laid on a wet coating. Those among the colors which require a dry, cretaceous, coating, and refuse to adhere to a wet surface, are purpurissum, indicum, caeruleum, melinum, orpiment, appianum, and ceruse.” Pliny, Natural History, book 35, chap. 31.
2. The Collected Works of Sir Humphrey Davy, edited by John Davy, 1839, 9 vols., vol. 6, p. 139.
3. Pliny, Natural History, book 35, chap. 6.
4. Pliny, Natural History, book 34, chap. 54
5. Eraclius, De Coloribus et Artibus Romanorum, in Mary Philadelphia Merrifield, Original Treatises: Dating from the XIIth to XVIIIth Centuries on the Arts of Painting, in Oil, Miniature, Mosaic, and on Glass; of Gilding, Dyeing, and the Preparation of Colours and Artificial Gems, 1849, vol. 1, p. 236.
6. Eraclius, De Coloribus et Artibus Romanorum, in M. Merrifield in Original Treatises, etc., vol. 1, p. 194.
7. Mappae Clavicula. Clarke MS 2260.
8. Theophilus, Diversarum artium schedula, chap. 37, found in On Divers Arts: The Foremost Medieval Treatise on Painting, Glassmaking, and Metalwork By Theophilus. Translated by John G. Hawthorne, Cyril Stanley Smith, 1979, p. 41–2.
9. The title of this treatise is The Guide to Painting by Dionysius, a monk of Fourna, translated by M. Didron and Dr. Paul Durand, Manuel d’Iconographie Chrétienne, Grecque et Latine, Paris, 1845, pp. 34, 48. This manuscript is probably a copy of an older MS, compiled by the monk Dionysius from the works of Manuel Pauselinos, of Thessalonica, a painter who worked in the twelfth century. See also Paul Hetherington, The Painter’s Manual of Dionysius of Fourna, Oakwood Publications, 1974. p. 11.
10. Petrus de Sancto Audemaro, De Coloribua Faciendis, in M. Merrifield, Original Treatises, vol. 1, p. 120.
11. M. Merrifield, Original Treatises, etc., vol. 2, p. 326, 484.
12. M. Merrifield, Original Treatises, etc., vol. 2, p. 770.
13. British Museum, MS. Sloane 122, f. 92v.
14. Daniel V. Thompson, The Materials of Medieval Painting, 1936, pp. 92-93.
15. Sir Philiberto Vernatti, “A Relation on the Making of Ceruss,” Philosophical Transactions, vol. 12, 1678, p. 935–36.