Benzimidazolone Yellow Pigment
Benzimidazolone Yellow is a high-performance green shade yellow benzimidazolone pigment, designed to have improved weatherfastness and higher tint strength than pre-existing Pigment Yellow 151 grades.
Benzimidazolone Yellow is a high-performance green shade yellow azo pigment designed to have improved weatherfastness and higher tint strength than pre-existing Pigment Yellow 151 grades. It has good gloss retention, tinting strength, and enhanced outdoor durability. It is considered a coloristic match with better weather resistance and the highest chroma compared to competitive grades. This yellow azo pigment has been registered according to the REACH regulation. It is on the following chemical inventories: US TSCA, EU, Japan, Australia, Korea, China, Canada, Philippines, New Zealand, and Taiwan.
| Pigment Information | |
| Color: | Yellow |
| Pigment Classification: | Synthetic Organic |
| Colour Index: | Pigment Yellow 151 (13980) |
| Chemical Name: | Benzimidazolone (monoazo, acetoacetyl, H4G) |
| Chemical Formula: | C18H15N5O5 |
| Chemical Structure: | 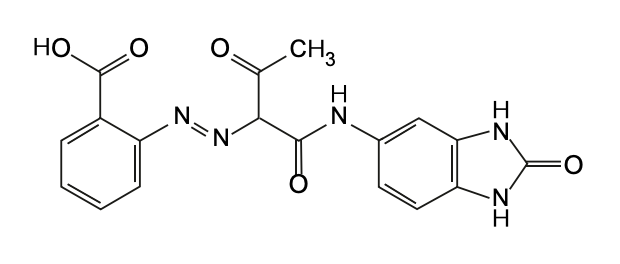 |
| CAS No.: | 31837-42-0 |
| Series No.: | 4 |
| ASTM Lightfastness | |
| Acrylic: | I |
| Oil: | I |
| Watercolor: | I |
| Physical Properties | |
| Specific Surface: | 0.9 m2/g |
| Density: | 1.6 g/cm3 |
| Bulk Volume: | 16 l/kg |
| Refractive Index: | 1.721 |
| Oil Absorption: | 52 ml oil / 100 grams pigment |
| Health and Safety | No acute or known chronic health hazards are associated with this product’s anticipated use (most chemicals are not thoroughly tested for chronic toxicity). Always protect yourself against potentially unknown chronic hazards of this and other chemical products by keeping them out of your body. Do this by avoiding ingestion, excessive skin contact, and inhaling spraying mists, sanding dust, and vapors from heating. Conforms to ASTM D-4236. |
For a detailed explanation of the terms in the table above, please visit Composition and Permanence.
Origin and History
The first azo pigment, tartrazine yellow, PY100, still used in artists’ paints, was patented in 1884. β-naphthol azo pigments were introduced from 1895 to 1911 and are still in production. The first Hansa yellow, PY1, was patented in 1909, and the first diarylide was synthesized in 1911. Because their importation from Germany was forbidden during World War I, American manufacturers began to study azo dyes in depth. Benzimidazolone pigments were first prepared in 1960, the same year the diazo condensation pigments were introduced to the United States. Azo pigments have become the largest class of synthetic organic pigments because of their ease of preparation and wide range of colors.
| Pigment Names | |
| Common Names: | Aureolin (hue) |
Source
Benzimidazolone pigments are monoazos containing benzimidazolone moiety as part of the coupling component. As the benzimidazolone ring of the coupling component imparts significant lightfastness, several pigments have been synthesized to provide a broad color range for this subclass. The acetoacetylarylamides range from greenish-yellow to orange, whereas the Naphthol AS types are red, carmine, violet, and brown.
Permanence and Compatibility
Benzimidazolone pigments have outstanding bleed resistance, lightfastness, and moderate heat resistance. The lightfastness of some benzimidazolones approaches that of quinacridones and phthalocyanine. Benzimidazolones also possess high heat stability (usually to 260°C, although some members of this class are stable only to 200°C); some are among the most heat-stable organic. The general structure of diazo condensation pigments: (a) yellow series and (b) brown, red, and violet series pigments. Benzimidazolones are used in artists’ paints, for coloring plastics, automotive coatings, and colored pencils and wood stains.
Oil Absorption and Grinding
Benzimidazolone yellow absorbs a large amount of oil—for every 100 grams of benzimidazolone yellow, 52 grams of linseed oil is needed to form a coherent paste, the oil absorption value for this pigment.
Toxicity
Benzimidazolone yellow is not considered toxic; however, care should be used in handling the dry powder pigment to avoid inhaling the dust. All toxicological studies showed no signs of toxicity to humans or the environment.
For more information on handling pigments, please visit How to Safely Handle Art Materials and Pigments.
Ecology
Benzimidazolone yellow is not among organic pigments that contain inadvertent polychlorinated biphenyls (PCB) or dichlorobenzidine (DCB) compounds.
| SKU | 436-12 |
|---|---|
| Brand | Rublev Colours |
| Vendor | Natural Pigments |
| Processing Time | Usually ships the next business day. |
| Color | Yellow |
| Pigment Type | Organic, Synthetic |



