
Studies of the reaction of painting supports, oils, and pigments to changes in the environment during the past hundred years make it possible to understand the behavior of paintings. Modern commercial oil paints present new issues to conservators as they observe defects in paint films caused by new pigments and additives used in their formulations.
In recent years, we have witnessed an increased interest among artists in historical painting techniques and materials. This trend is subject to much speculation, but some may say it is due to the revival of figurative art. I prefer to say that interest in figurative art never really died out, but rather it has survived abstract art.
An essential part of this interest in materials is a concern for the longevity of the paintings created by contemporary artists, a care observed by the old masters in their work.
Cracked, cupped, and blistered paint attests to the presence of considerable mechanical forces in paintings. Not until recent years was it believed that the movements of the canvas or panel were the primary cause of paint cracking and deterioration. This led early investigators to concentrate on the behavior of the support. Their studies of the reaction of painting supports to changes in the environment made it possible to understand the behavior of paintings.
The Importance of Supports
Changes in temperature and relative humidity cause the materials of a painting to contract and expand. The rate of expansion and contraction is at different rates, one for the canvas or panel, another for the size, and yet another for the ground and paint film.
The rate of expansion and contraction is even different for different pigments in a paint film. The schematic shows a more flexible paint segment bound by stiffer segments. (Berger 1994)
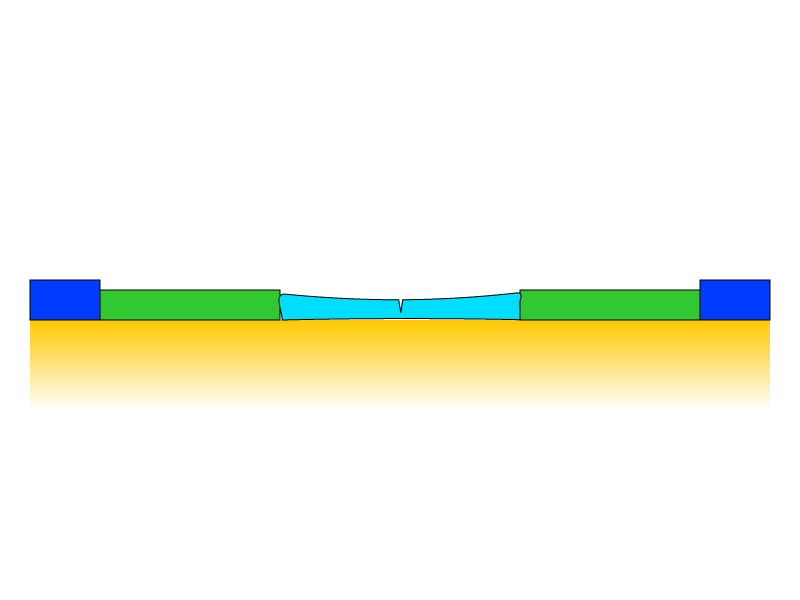
How cracks develop in the paint from compression and expansion forces caused by relative humidity and temperature cycles.
In the example above, temperature and relative humidity changes cause disproportionate compression of the central segment, which occurs during contraction and expansion cycles. In the contraction phase, the center segment is stretched, and the segment shows early signs of mechanical failure. Another expansion cycle occurs, and upon return to its rest state, the paint layer does not recover its elasticity due to compression and expansion cycles, and a crack develops.
If the canvas is to support the rest of the painting, it should be stiff and strong. Ideally, the size, ground, and paint supported by the canvas should be more flexible than the support. However, the opposite is true, particularly at low relative humidity.
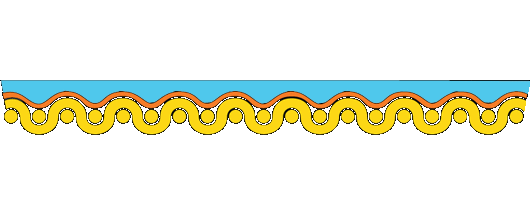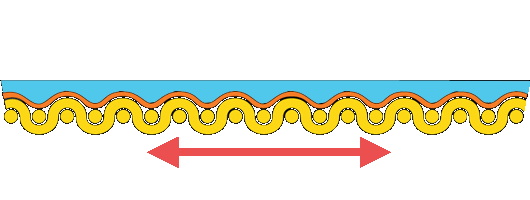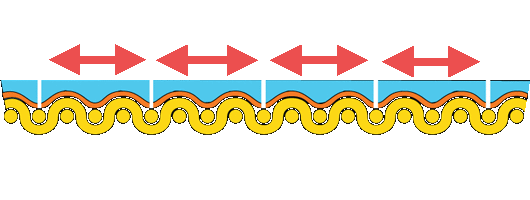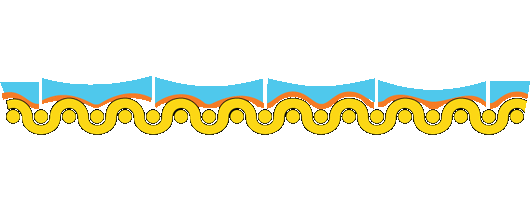
Paintings on stretched canvas deteriorate because each component of the painting responds differently to environmental changes. Studies show that much of the tension is carried in the paint and ground, not the canvas.
Re-alignment of the forces in the painting causes the paint to cup and separate from the canvas. We can demonstrate the disparity in the stiffness of materials in a painting on canvas by dipping a rubber ball in plaster. As the plaster dries, it becomes stiff and inflexible, yet the rubber ball remains plastic, and when deformed, the plaster cracks and falls away.
Here we can see the combination of crack patterns caused by cycles of significant changes in relative humidity and stretcher expansion. (Mecklenburg 2007)
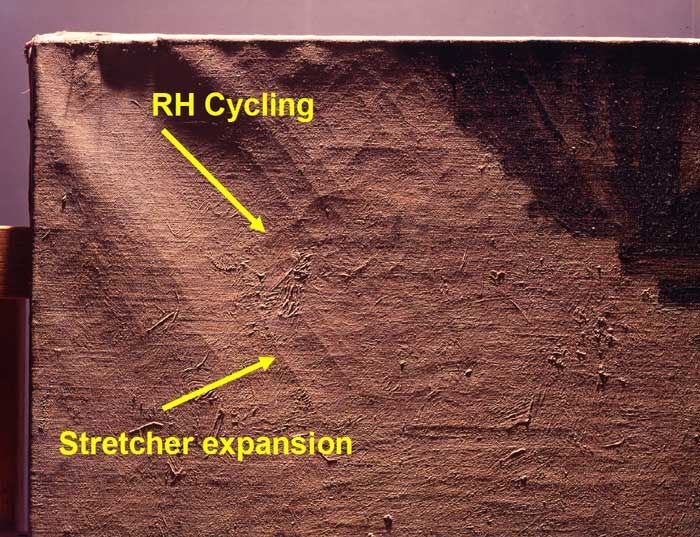
Paint film cracking is caused by relative humidity cycling and stretcher expansion. (Photo by Marion Mecklenburg)
Researchers found that the paint film and its support become a single composite structure in which all components interact. Hence, paint on canvas behaves completely differently from that on a rigid support. Thus a painting might crack on one type of support but not another. Paintings on rigid supports, such as by Flemish-born painter Gerard Van Spaendonck, painted on marble in 1800, exhibit minor mechanical failure.

Gerard Van Spaendonck (1746-1822) Bouquet of Tulips, Roses and an Opium Poppy, with a Pale Clouded Yellow Butterfly, a Red Longhorn Beetle and a Seven-spotted Ladybug. Oil on marble.
Painting on a rigid support with greater stiffness than the paint layer can prevent cracking and paint failure.
Rigid supports such as wood provide greater stability for paint films than canvas. However, wood is hygroscopic, meaning that it readily absorbs water and hence is affected by changes in relative humidity. Engineered wood products, such as plywood, fiberboard, and hardboard, provide greater stability due to the ply construction or random orientation of the wood fibers, yet are still susceptible to environmental changes.
To understand the amount of stress generated by different components of a painting, we will use a graph to show the percentage of swelling or shrinkage unrestrained material experiences when responding to changes in temperature or humidity. By ‘unrestrained,’ we mean that the material is not inhibited from expanding or contracting.
For example, a piece of loose fabric or wood that swells and shrinks as the humidity in a room changes. By measuring these dimensional changes, we can know how responsive the material is. Now, if this same material is restrained in some way, such as stretched canvas, we can sense how the tension will rise and fall as the canvas tightens and slackens, even though its outer dimensions stay the same.
In this way, the measurement of free swelling strains is directly related to the amount of stress generated in a painting whenever there are changes in environmental conditions.

The free swelling strain of linen due to changes in relative humidity. (Mecklenburg 2007)
If linen is free to expand and contract, it changes dimensions with changes in relative humidity. What is important regarding the dimensional response of linen to relative humidity is that while it is expected to contract when it dries, above 80% relative humidity, it also contracts.

The free swelling strain of scotch pine due to changes in relative humidity. (Mecklenburg 2007)
The free swelling of wood with changes in relative humidity is orthotropic, meaning that a particular cut of wood expands and contracts in two planes perpendicular to each other. Wood swells much more in the cross-grain tangential direction than in the longitudinal direction parallel with the grain. (Mecklenburg 2007)
Hide glue is only stable between 30% and 60% relative humidity. Oil paints, however, respond very little to changes in relative humidity. As a composite structure, the free swelling strains of this structure used in oil painting are diverse. (Mecklenburg 2007)

The free swelling strain of materials in an oil painting due to changes in relative humidity. (Adapted from Mecklenburg 2007)
An excellent way to visualize the composite nature of painting materials is by the swelling of the materials of a painting on a wood panel. Because a wood panel is thick relative to the other layers, its response determines the dimensional change in the other layers. At high relative humidity, the wood expands faster than the other layers and stretches the other layers. At low relative humidity, it shrinks, compressing the gesso and paint layers. (Erhardt 1995)
On the other hand, metals such as copper do not change with relative humidity changes. However affected by changes in temperature, these changes are reduced by composite structures, such as aluminum composite materials (ACM). ACM comprises two sheets of aluminum bonded to a polystyrene core or through structural improvements like aluminum honeycomb panels where a honeycomb core is covered on both sides with an aluminum skin. Materials such as these can prevent much cracking and paint loss found on stretched canvas supports and wood panels.
What is Oil Paint?
Oil paint consists of two basic components: pigment for color and a binder to act as a glue. These interact to form a composite, or we may think of them as a system.
The primary function of the binder is to carry and bind the pigment. For the paint film to be cohesive, the pigment must be enveloped within the binder. Second, to impart working characteristics. It should allow the painter to manipulate the paint consistently. Third, to secure the color to the surface in a stable and long-lasting manner. A well-dispersed pigment within a quality binder should dry without wrinkling, cracking, or buckling as long as it’s applied with conscientious technique.
One way to think about the relationship between pigment and binder is a brick wall. Every mason knows there is an ideal ratio of mortar to brick. Too much mortar, and the wall is weak. Not enough mortar, and the bricks fall apart.
The same relationship exists between the pigments and binder in dried paint. We call this relationship or ratio the pigment volume concentration or PVC. Pigment volume concentration (PVC) is the volume of pigment compared to the volume of all solids. If the paint has a PVC of 30, then 30% of the total binder/pigment is pigment, and 70% is binder solids.
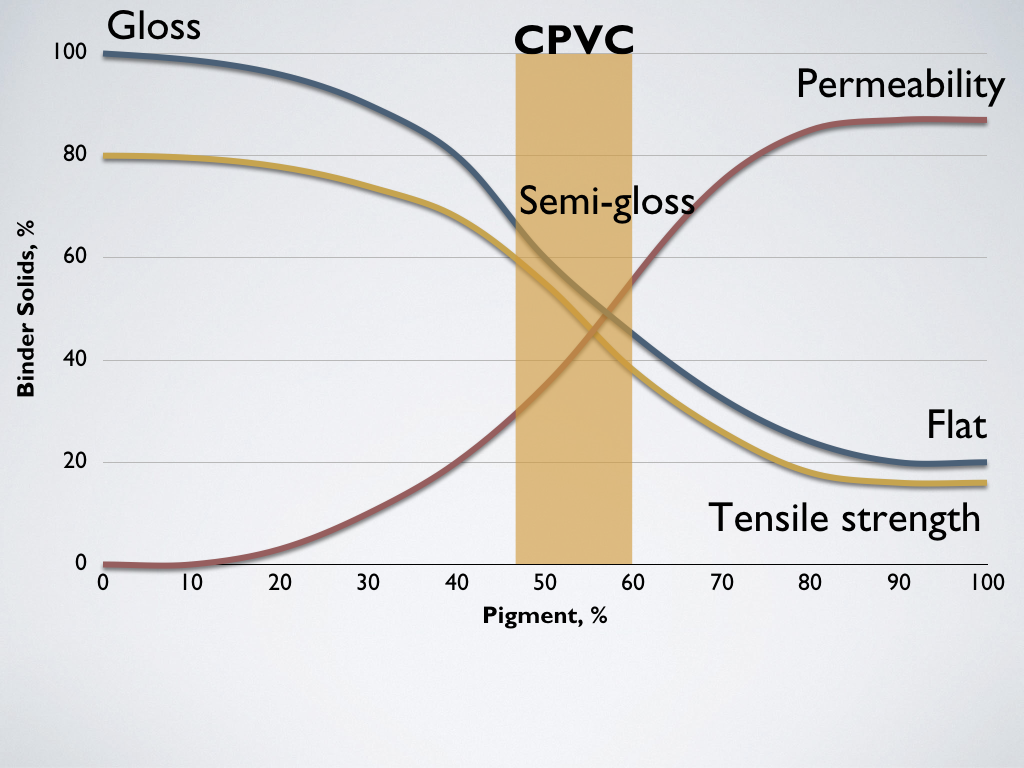
Paint film properties based on Pigment Volume Concentration (PVC)
The point at which there is just enough binder to wet pigment particles is called the critical pigment volume concentration (CPVC). This is between 45% and 55% PVC for almost all colors. Films with lower pigment concentrations have more gloss, but as the PVC increases, they become increasingly matte. Films with high percentages of pigment are more permeable to moisture and susceptible to solvents. This is because, with more pigment, there is less binder to fill the voids between pigment particles. This porosity leaves the film open to the environment. Films with higher pigmentation have increasingly lower tensile strength.
The volume of oil and pigment for oil colors is based on the Critical Pigment Volume Concentration (CPVC)
Most paints in tubes contain enough binder to wet and envelope pigment particles and are at about the critical pigment volume concentration for that color.
Fat-Over-Lean Rule
You are probably familiar with the fat-over-lean rule. What the fat-over-lean rule implies is flexible over less flexible paint layers. When you apply a more flexible layer on top of another, the final paint film will be more resilient and more resistant to cracking. Increasing flexibility is accomplished by adding more medium or oil or lowering the pigment volume concentration of the paint with each successive paint layer.
However, this isn’t easy to achieve in practice. Most artists don’t understand what constitutes fat or what is lean. It is impractical to measure the ratio of pigment and binder while painting. What I will tell you next may sound like heresy, but you do not need to follow this rule if you use paint at its CPVC, which is applying paint straight from the tube and thinly. On the other hand, working in thick paint layers or with lots of medium or oil added to paint requires one to observe the fat-over-lean rule.
Issues with “Traditional” Oil Painting Mediums
Perhaps the most common question artists ask is what medium they should use in their oil painting. We do not have enough time to answer that question correctly, but I would like to discuss resin-based mediums. Resins-based mediums are made with resin dissolved in a solvent, including dammar gum, popularized as a medium in the last century by Ralph Mayer, and mastic gum, used in a medium known as megilp, continues to be used by many artists in Maroger medium.
The use of resinous mediums in oil paint is certainly not new. However, the use of resins mixed with oil paint did not get widespread use until the latter half of the eighteenth century. Sir Joshua Reynolds was notable among artists using such mediums whose influence swayed generations of artists to incorporate resinous mediums in their work. Artists who use mediums in their paintings often claim that paint films incorporating such mediums show no signs of deterioration. Whether one accepts these claims or not, an important consideration that appears to have been overlooked is the solubility of the dried paint film and what happens during varnishing and cleaning paintings.
For centuries it has been common practice for artists or someone else to apply a coating of varnish to paintings. Such varnishes became darker, more yellow, and obscure within a few decades of application. It has been common practice to clean paintings at intervals, removing the darkened and obscuring varnish.
The solvents typically used to apply a varnish are largely benign to an oil painting so that the varnish can be applied without adverse effects on the paint. The resins used in varnishes can only be removed by the dissolving action of organic solvents.
The task in cleaning then is to use solvents that will remove the varnish but not damage the painting. The primary concern is that paint might be removed during cleaning. Another risk that has received considerable attention lately by conservation is “teaching,” the potential for soluble compounds in the paint to be extracted during cleaning. The loss of these components has been linked to the embrittlement of the paint film.
If the painting contains dammar or mastic resin in the paint film, cleaning or removing the varnish may swell and soften the resins in the paint layer, resulting in damage or, worse, the removal of thin paint layers containing these resins.
Differences Between Traditional and Modern Oil Paints
With the introduction of synthetic painting media, artists’ oils are, in most cases, still considered “traditional” painting materials and often mistaken for them. On the contrary, oil paints have experienced new production methods since the late eighteenth century. Paintings produced in the last 60–80 years generally suffer deterioration phenomena never or rarely observed in traditional oil paintings. In particular, twentieth-century oil paintings have conservation problems related to the fragility and the sensitivity of the paint layers. These phenomena can be understood in relation to two main reasons:
• The artist does not know what’s in the paint.
• The artist does not understand how additives and pigments used in modern oil paints interact and react with the environment.
While the artist was aware of the materials and often prepared them in the past, from the late nineteenth century, artists generally did not know exactly what they used in their paintings. In the twentieth century, the use of lead white diminished because of toxic concerns, and titanium white was introduced as a replacement white. The problem is that oil paints dry to fragile and somewhat brittle films using titanium dioxide.
Manufacturers of artists’ materials introduced zinc oxide into titanium white paints to give them additional strength. Paints made with only zinc oxide not only became more available to artists, but most of the lead white paints often contain zinc white. These paints can become extremely brittle in as little as three years of drying, even under “ideal” museum conditions. In addition to white paints, many of the light colors labeled “hues” also contain zinc oxide, and they can potentially become equally brittle. (Mecklenburg 2007, Maachia 2015, Osmond 2012, 2014)

Detail of Henry Cliffe’s painting of 1959 showing cracking of lead white and zinc white paint (Mecklenburg 2007)
This is a detail of an abstract expressionist painting from 1959 by Henry Cliffe. The oil paints used in this painting contain lead white and zinc white. This painting was rolled, and the damage was extensive. In addition to the severe cracking, there is extensive interlayer cleavage. (Mecklenburg 2007)

The layer of zinc oxide oil paint delaminating from the acrylic ground. (Photo by Marion Mecklenburg)
Paints containing zinc oxide also bond poorly to adjacent layers of paints and grounds in paintings. Although oil paints generally adhere well to acrylic grounds. In the case of the lead white containing zinc oxide, the paint could be laminated from the acrylic ground. In this sample, the layers separated from each other cleanly. (Mecklenburg 2007, Maor 2008)

Carbon black oil paint exhibits widespread cracking over zinc oxide in Franz Kline, Palladio, 1961. (Rogala 2010)
Here is an example of cracking of carbon black painted over zinc oxide oil paint on Franz Kline’sspainting Palladio, completed in 1961. In the same painting, the carbon black paint remains intact where it is painted over lead white but exhibits widespread cracking over zinc white paint. (Rogala 2010)
Zinc oxide is very reactive in drying oils, forming zinc soaps in paint films. Zinc white pigment particles are so small that they can react away, diminishing their light reflectance. This is seen in the figure of the father in Luke Fildes’’1890 painting of The Doctor. The father appears to be fading away in the background caused by the chemical and physical changes to zinc oxide. (Shimadzu 2008)

The father painted with zinc oxide is gradually fading in Luke Fildes’’1890 painting The Doctor.
Oil paints are complex because different pigments cause paints to dry with very different mechanical properties. To understand how each component of the paint film behaves, we need to understand terms used in materials research. A stress-strain curve is one way to measure how strong and flexible the material is and when it is liable to break.

Stress-strain curve (Adapted from Sands 2011)
In her article “Using Oils with Acrylics,” Sarah Sands explains how we can understand the relative flexibility of materials by looking at their stress-strain curve: “In the diagram, the curved line represents the behavior of many materials when being stretched. At first, the line rises steeply as the force required to overcome the initial resistance to stretching increases and begins to elongate. The breaking point of the materials is represented at the end of the line. The amount of force needed to elongate the material is called the “tress,” while the percentage the material stretches is known as the “train.’” “Another important concept is called the “yield point.” This is the point when the material is stretched so far that it can no longer return to its original shape and so is permanently deformed or damaged. For most artist materials and supports, this is less than one percent (0.5%) and shows just how little strain paintings can endure.” (Sands 2011)
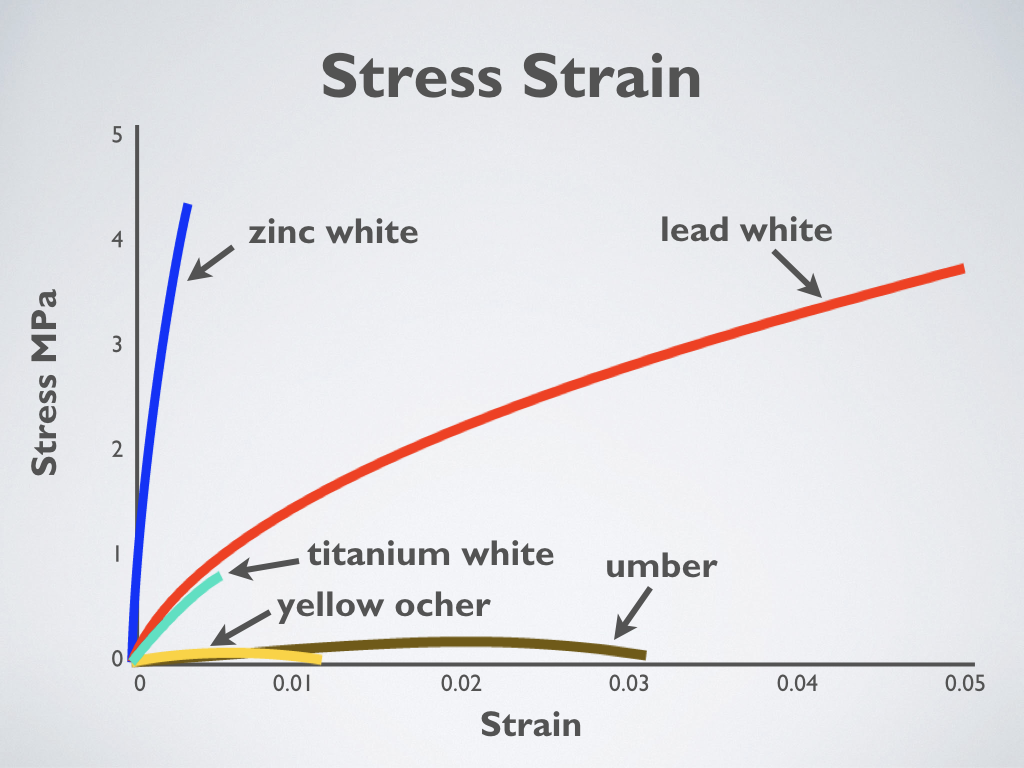
Stress-strain of oil paint shows relative flexibility of different colors (Adapted from Mecklenburg 2007)
The graph shows the stress-strain results for different oil paints after drying for at least 12 years in a controlled environment. Paints made with earth colors develop little strength but remain flexible. Paints with titanium white and lead white have nearly the same initial strength, but the titanium white is weak and breaks readily. Titanium white can be considered a weak and brittle paint. Paint made with zinc oxide has very high strength but is a very brittle paint.
It is necessary to understand that while the strength of paint is essential, its ability to stretch (its flexibility) is of far greater importance. It doesn’t take much force to crack thin paint films even though they have relatively high strength. Paints with titanium white and lead white have nearly the same initial strength, but the titanium white is weak and breaks readily.
Traditional and Modern Drying Oils
Besides the traditional drying oils of linseed, poppy seed, and walnut oil, other drying or semi-drying oils are employed in modern oil paints, sometimes as cheap substitutes for traditional drying oils. Although dried, semi-drying oils applied in thick layers have been observed to “weep’ on modern oil paintings. Here is an experiment with a cadmium hue mixed with sunflower-safflower oil weeps five years after the paint dried. (Boon 2014)

A tube of Norma Fleischfarbe pink paint (a) used by van Hemert for his Seven series paintings. The paint was used in 2002 for thin and thick paint application (b) on nonpermeable support (Photos were taken in 2005 and 2010). In late 2009 the thicker impasto swatch (c) showed a molten appearance followed by drip formation (Boon 2014).
Additives in Modern Oil Paints
Among the changes to modern oil, paints have been the use of dispersing agents and stabilizers. The first to be used in modern oil formulations were aluminum stearate. This metal soap was first used in artists’ paints in the early twentieth century. (Tumosa 2001) Since that time, other agents have been used in modern oil paints, such as magnesium stearate, zinc stearate, and waxes, such as castor wax.
The addition of stabilizers keeps the pigments in suspension, prevents oil and pigment separation, prolongs shelf life, and provides uniform handling properties of the paints in tubes. Although these additions to oil paints perform these functions well, they also reduce the cost of oil paint production, creating a cheaper paint since lower amounts of pigments are needed.
Many other additives have been included in oil formulations in modern manufacturing processes. Resins, fillers, surfactants, and other adulterants in different amounts are quite common, especially for less expensive paints. Unfortunately, industrial secrets do not permit one to know about adding these materials, and labels on tube oils are vague. (Izzo 2010, Fuster-López 2016)

Haze on painting caused by efflorescence
A phenomenon observed in modern paints is a milky haze on the surface of the paint film. After aging, superficial efflorescences are observed in modern paint films, particularly those with higher proportions of additives. This phenomenon is likely the effect of the migration to the surface of additives, such as stearates. (Schilling 1998, 1999; Ordonez 1998, Akerlund 2013)
Conclusion
While the development of modern pigments and synthetic resins has resulted in paints with greater lightfastness and stability, the use of zinc oxide, semi-drying oils, stabilizers, and other additives has not been without their issues. In review, the issues include:
• Zinc oxide creates brittle oil paint films and can cause superficial layers to crack and delaminate
• Zinc oxide reacts away, causing color changes
• Semi-drying oils may not polymerize completely, resulting in “weeping” paint.
• Stearates and waxes can cause efflorescences
|
Painting Best Practices
The complete presentation by George O'Hanlon, founder and director of Natural Pigments, on the best practices in painting was given at the Figurative Art Convention & Expo (FACE) in Miami on Friday, November 10, 2017. The lecture is based on studies of the reaction of painting supports, oils, and pigments to changes in the environment during the past hundred years making it possible to understand the behavior of paintings. Modern commercial oil paints present new issues to conservators as they observe defects in paint films caused by new pigments and additives used in their formulations.
This article presents a small portion of the information presented in the three-day Painting Best Practices workshop offered by Natural Pigments. This information is also available on the Painting Best Practices website.
Sources
Akerlund, Luciana. Efflorescence in Paintings and the Role of Moisture. Collectie Wijzer. January 18, 2013. Blog.
Berger, Gustav A. and William H. Russell. Interaction Between Canvas and Paint Film in Response to Environmental Changes. Studies in Conservation, Vol. 39, 1994, pp. 73–86.
Boon, Jaap J., F. G. Hoogland. Investigating Fluidizing Dripping Pink Commercial Paint on Van Hemert’s Seven-Series Works from 1990–1995. In: van den Berg K. et al. (eds) Issues in Contemporary Oil Paint. 2014. Springer.
Erhardt, David, Marion F. Mecklenburg, Charles S. Tumosa, Mark McCormick-Goodhart. The Determination of Allowable RH Fluctuations. WAAC Newsletter, Vol. 17, No. 1, January 1995, p. 19.
Fuster-López, Laura, Francesca Caterina Izzo, Marco Piovesan, Dolores J. Yusá-Marco, Laura Sperni, Elisabetta Zendri. Study of the Chemical Composition and The Mechanical Behaviour of 20th Century Commercial Artists' Oil Paints Containing Manganese-Based Pigments. Microchemical Journal 124. 2016. pp. 962–973. Paper.
Izzo, Francesca Caterina. 20th Century Artists' Oil Paints: A Chemical-Physical Survey. Thesis. 2010. pp. 45–50. Paper.
Macchia, Andrea and Stella Cesaro, Yeghis Keheyan, Silvestro Ruffolo, Mauro La Russa. White Zinc in Linseed Oil Paintings: Chemical, Mechanical and Aesthetic Aspects. Periodico di Mineralogia. 83. 2015. 30-43. 10.2451/2015PM0027. Paper.
Maor, Yonah. Delamination of Oil Paint from Acrylic Grounds. Thesis. Queen’s University, Kingston, Ontario, Canada. September 2008. Paper.
Mecklenburg, Marion F. and Charles S. Tumosa. The Chemical and Mechanical Effects of Pigments on Drying Oils. 2007. pp. 13–14.
Mecklenburg, Marion F. Determining the Acceptable Ranges of Relative Humidity And Temperature in Museums and Galleries, Part 2, Structural Response to Temperature. Museum Conservation Institute, 2007. pp. 3, 47. Paper.
Mecklenburg, Marion F. and Charles S. Tumosa. The Chemical and Mechanical Effects of Pigments on Drying Oils. 2007. pp. 13–14.
Mecklenburg, Marion F. Microclimates and Moisture Induced Damage to Paintings’ in Padfield, T., Borchersen, K. eds., Museum Microclimates. Contributions to the conference in Copenhagen 19-23 November 2007, National Museum of Denmark, Copenaghen. pp. 21–25. Paper.
Mecklenburg, Marion F.; Charles S. Tumosa, Edward P. Vicenzi. The Influence of Pigments and Ion Migration on the Durability of Drying Oil and Alkyd Paints. 2008. Museum Conservation Institute. Paper.
Ordonez, Eugena and John Twilley. Clarifying the Haze, Efflorescence on Works of Art. WAAC Newsletter Vol. 20, No. 1. January 1998. Paper.
Osmond, Gillian. Zinc White: A Review of Zinc Oxide Pigment Properties and Implications for Stability in Oil-Based Paintings. AICCM Bulletin. Vol. 33, 2012.
Osmond, Gillian. Zinc White and the Influence of Paint Composition for Stability in Oil Based Media. In: van den Berg K. et al. (eds) Issues in Contemporary Oil Paint. 2014. Springer.
Rogala, Dawn, Susan Lake, Christopher Maines and Marion Mecklenburg. Condition Problems Related to Zinc Oxide Under Layers: Examination of Selected Abstract Expressionist Paintings from the Collection of the Hirshhorn Museum and Sculpture Garden, Smithsonian Institution. Journal of the American Institute For Conservation, Vol. 49, No. 2 (Fall/Winter 2010), pp. 96–113.
Sands, Sarah. Using Oils with Acrylics. Just Paint, Issue 24, 2011. Golden Artists Colors. Paper.
Schilling, Michael R., David M. Carson, and Herant P. Khanjian. Evaporation of Fatty Acids and the Formation of Ghost Images by Framed Oil Paintings. WAAC Newsletter, Vol. 21, No. 1. September 1998. pp. 1–5. Paper.
Schilling, Michael R., David M. Carson and Herant P. Khanjian. Gas Chromatographic Determination of the Fatty Acid and Glycerol content of Lipids. IV. Evaporation of Fatty Acids and the Formation of Ghost Images by Framed Oil Paintings. In Proceedings of the 12th Triennial ICOM-CC Meeting, Lyon, 29 August-3 September 1999. Vol. I. pp. 242–247.
Shimadzu, Y., K. Keune, K. J. van den Berg, Jaap J. Boon, J. H. Townsend. The Effects of Lead and Zinc White Saponification on Surface Appearance of Paint. ICOM Committee For Conservation, 2008 Vo. II, p. 626.
Tumosa, Charles S. A Brief History of Aluminum Stearate as a Component of Paint. WAAC Newsletter. Vol. 23 No. 3. September 2001. p. 1. Paper.
Tumosa, Charles S., and Marion F. Mecklenburg. The Influence of Lead Ions on the Drying of Oils. Reviews in Conservation, 2005: 6:39–47. Paper.












