In recent years, we have witnessed the trend towards using traditional artists’ materials—painting materials used before the twentieth century. This is evident from the growth of small artists’ color companies and the increased interest among artists in historical painting techniques and materials. Why this trend is occurring is subject to much speculation, but some may say it is due to the revival of figurative art, especially since the 1970s. I prefer to say that interest in figurative art never really died out, but rather it has survived abstract art.
Suppose this is the case, that the trend towards traditional artists’ materials is related to the renewed interest in figurative art. In that case, Bill Creevy, author and contributing writer to American Artist magazine, may have identified the motivation for this trend. He said, “The advantage to being a figurative artist is that you don’t have to worry about being in or out of fashion. All you have to worry about is whether you can pull it off.”
This implies that a figurative artist must be skilled to ‘pull it off.’ The idea of skill in painting has always been associated with the old masters. The development of drawing and painting skills may have stimulated the trend toward traditional artists’ materials.
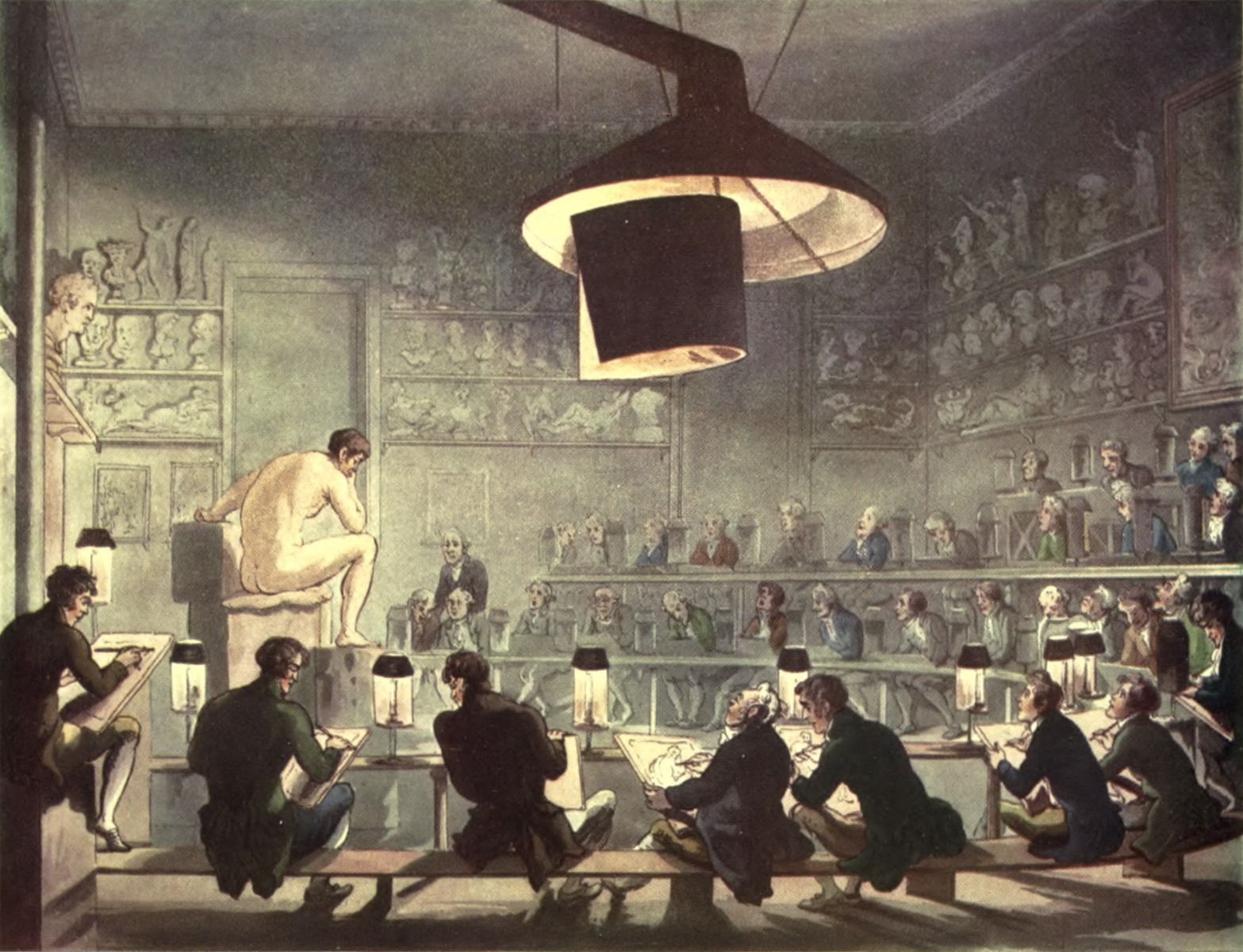
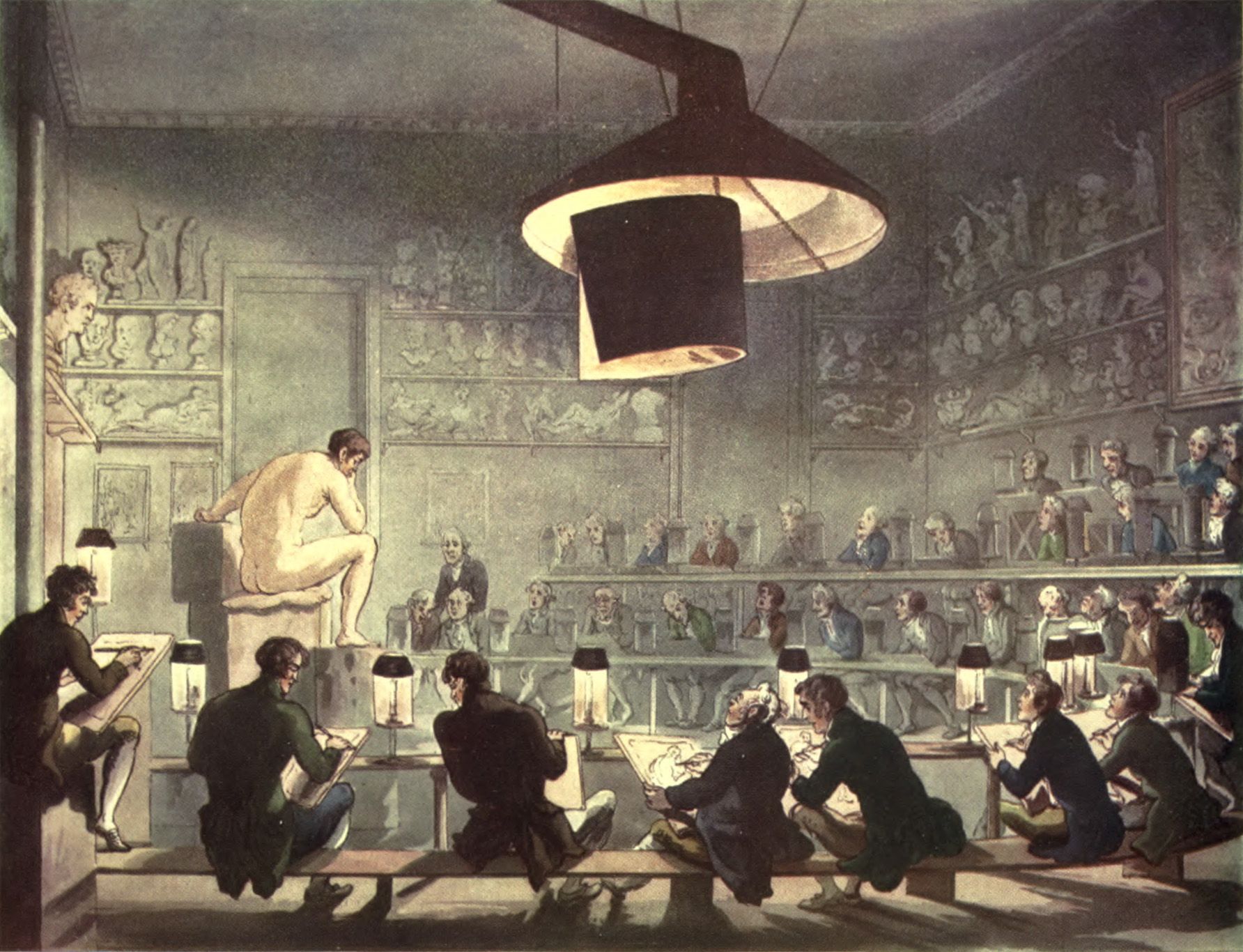
Fig. 1 Drawing from Life at the Royal Academy, 1808, Pyne, William Henry and Combe, William “Royal Academy,” in The Microcosm of London or London in Miniature (Volume I ed.). London: Methuen and Company. pp. Plate 1.
This article examines traditional oil paints, what makes them different from modern colors, and what benefits they provide artists today. To understand traditional oil paints, we first need to examine how they were made.
During the Middle Ages and the Renaissance, the paint used by artists was prepared in the studio. The painter purchased pigment from apothecaries and apprentices, who also prepared panels and grounds for the master painter, then prepared it for use as paint. To obtain a smooth-spreading paint, the pigments had to be ground into reasonably uniform-sized particles. Most pigments were ground as smoothly as possible to improve their color and to make a better flowing paint. The pigment was mixed with sufficient medium to make an easily workable paint. The recipes or instructions used by painters were handed down from master to pupil. Many survive as manuscripts and printed books, such as Theodore de Mayerne’s seventeenth-century notebooks on painting and Cennino Cennini’s fourteenth-century treatise, Il Libro dell’Arte.

Fig. 2 A sixteenth-century artist’s workshop with apprentices (right) grinding paint from Color olivi, Colorem olivi commodum pictoribus, Inuenit insignis magister Eyckius, Jan Baptist Collaert after Johannes Stradanus, 1591, Copper engraving by an anonymous engraver, 20.5 x 27 cm. Nova Reporta.
The oil paint used by artists from the fifteenth to nineteenth centuries consisted primarily of pigment and vegetable oil. However, sometimes gums, proteins, and resins were added for particular passages in a painting. Preparing oil paint involved mixing oil, typically linseed or walnut oil, with pigment previously prepared by merchants or artists’ apprentices. The pigment and oil were mixed on a flat stone slab into a smooth paste with a muller. The paint was then placed into shells for immediate use or in pigs’ bladders for later use.

Fig. 3 The artist’s apprentice (left) is preparing paint on a grinding slab from Emperor Maximilian I in the Artist’s Studio, 1512–16, Woodcut, 219 x 193 mm, From Der Weiss Kunig (Der Weiß Kunig), 1775 edition.
By the late seventeenth century, an area of trade had come into being, the artists’ colormen, who supplied prepared pigments, made brushes, prepared canvases, and other items required by the artist. The increasing amateur interest in painting may have stimulated the trade’s emergence. However, most professional painters continued to prepare their paints, possibly through fear of adulteration of pigments. Unscrupulous suppliers often adulterated expensive pigments like natural ultramarine (the pigment made from lapis lazuli) and vermilion with cheap additives.
Effects of Particle Size and Shape of Pigments
By the early nineteenth century, most of the colormen produced color from traditional pigments manufactured by traditional methods. Advances in the chemical industry at the close of the eighteenth century and throughout the nineteenth century produced an enormous expansion in the range of pigments. Some of these new pigments made valuable additions to the artists’ color range by providing less expensive alternatives for expensive traditional pigments, for example, artificial ultramarine. For the first time, synthetic pigments replaced traditional pigments on the artists’ palettes. Although natural and synthetic ultramarine pigments are chemically similar, their hues and behavior in paint are remarkably different.

Fig. 4 Semi-precious lapis lazuli stone contains the blue mineral lazurite and accessory minerals.

Fig. 5 Lapis lazuli particle viewed in plain- (left), and cross-polarized (right) light clearly shows its variable composition of the blue mineral lazurite and accessory minerals.
Natural ultramarine consists of the blue mineral lazurite (10 to 50%), a feldspathoid silicate. It usually also contains calcite (white), sodalite (blue), and pyrite (metallic yellow) in the semi-precious lapis lazuli stone. (fig. 4) Other possible constituents are augite, diopside, enstatite, mica, and wollastonite. It is impossible to completely free the lazurite from the other minerals, as seen in the microphotographs. The fragment of lazurite viewed in cross-polarized light shows lazurite as blue and calcite as white patches. (fig. 5) When viewed in plain polarized light, pyrite and other minerals appear as dark spots.

Fig. 6 Natural ultramarine viewed in partially polarized light at 64 times magnification.
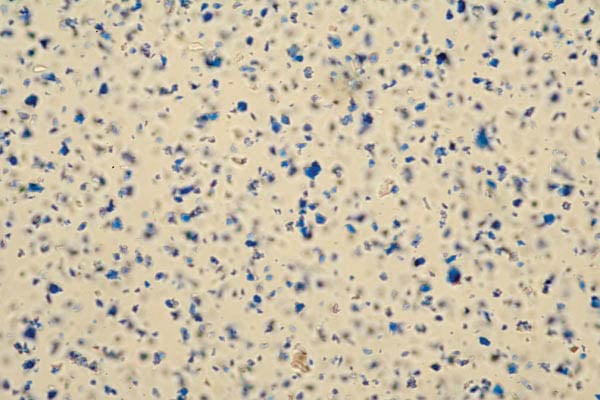
Fig. 7 Synthetic ultramarine viewed in partially polarized light at 64 times magnification.
Besides a difference in composition, another more critical difference lies in the pigment particles. The microphotograph (fig. 6) shows the diverse range of particle sizes and shapes of natural ultramarine pigment made from lapis lazuli. In contrast, synthetic ultramarine is a collection of very fine, homogenous particles. (fig. 7)

Fig. 8 Malachite pigment coarsely ground (1–150 microns) (left) and finely ground (1-60 microns) (right).
Pigment particles do not dissolve in the paint vehicle but remain as discrete particles. The size and shape of pigment particles greatly influence the consistency and behavior of paint. Pigments in older paintings, in general, are coarse, particularly mineral pigments, compared to pigments in artists’ colors today. Traditional mineral pigments, for example, malachite, azurite, and smalt, have to be used coarsely ground because, when very finely ground, so much white light is reflected from the surfaces of their particles, they become pale and unsuitable as coloring materials.[1]
The particle size of pigments used by artists’ colormen in the nineteenth century did not change much from previous centuries. According to the archives of Lewis Berger & Company, Ltd., a colorman supplying such firms as Winsor & Newton,[2] the smallest sieves used to screen pigments were 150-mesh. This size mesh retains particles over 104 microns, the smaller particles passing through the screen and presumably sold to paint manufacturers. This is different today, where modern pigments’ fine and uniform particle size is primarily the result of modern methods of manufacturing pigments.

Fig. 9 Sieves are used to screen out different-sized particles.
Mineral pigments, such as azurite, are ground from ores resulting in large, fractured crystals. Grinding a pigment in paint ordinarily does not reduce particle size but merely affects the wetting and dispersion of individual pigment particles. Artists’ color makers today do not manufacture the pigments they use in their paint, so they must rely on large industrial firms for their supply of pigments. Pigments intended for paint production are usually supplied in a particle size range such that nearly all pigment particles pass through a 325-mesh sieve. [3] This sieve size screens out particles larger than 44 microns.
Modern pigments are developed quantitatively for the paint industry, in which producing paints for artists plays an insignificant role. They are formulated for maximum tinting strength, covering power, and stability in paint without concern for their chromatic diversity and novel consistency. To achieve maximum desirability in modern paints, pigments are made homogenous in shape, size, and composition. For example, particle sizes are reduced to the smallest possible to increase the covering power of a pigment. The smaller the particles, the more the color nuances of the pigment are absorbed into its primary hue, as in inks with no texture. Particles are more consistent in shape and size and tend not to settle quickly and separate from their binder once inside a container. This increases the shelf life and, thereby, marketability of paint but does not necessarily increase its desirability as a color for artists.

Fig. 10 Relative size of different particles
To understand the relative size of particles in modern and traditional paints, we can compare it to an everyday object—typical human hair, which is about 50 microns thick. (fig. 10) A 100-micron particle of a mineral pigment in artists’ colors before the twentieth century would be twice the thickness of a strand of hair. The largest particles in modern colors are less than 44 microns, and typically the median particle size is less than 3 microns.

Fig. 11 Natural yellow ocher viewed in partially polarized light at 160 times magnification.

Fig. 12 Synthetic yellow iron oxide (Mars Yellow) viewed in polarized light at 160 times magnification.
The difference in particle size is not confined to rare mineral pigments. Yellow ocher is a pigment on every artist’s palette of every period in history. And although it is still widely available, the trend in recent years has been to replace it with synthetic yellow iron oxide while still naming the artists’ oil color yellow ocher. The difference between the two pigments is more than their composition; the microphotograph of yellow ocher shows particles of heterogeneous size and shape (fig. 11), while particles of yellow iron oxide are very uniform and fine (fig. 12). The particle size distinction creates paint with very different properties.
The difference between modern and traditional artists’ colors provided by the particle size and shape of pigments cannot be more appreciated than with lead white. Lead white is the most important white pigment in history. It is one of the earliest pigments to be made artificially, known to the Egyptians, ancient Greek artists used it in painting, and the Roman historian Pliny described how to prepare it. Its method of manufacture did not change significantly from Pliny’s description of it in the first century before the Common Era until the beginning of the twentieth century.
This method is known as the “Old Dutch method” or “stack process,” which consists of placing lead strips or castings in earthenware pots containing a small amount of vinegar. The pots are placed in layers of horse dung and left for up to three months. As the dung decomposes, it releases heat, water, and carbon dioxide, the essential ingredients in making lead white or basic lead carbonate. After some time, the lead is corroded, resulting in a white pigment that flakes off the metallic lead. Since the early twentieth century, the process of making lead white radically changed to the “quick process.” (For a detailed description of the Old Dutch method of lead white, see the article, Stack Process White Lead—Historical Method of Manufacture.)
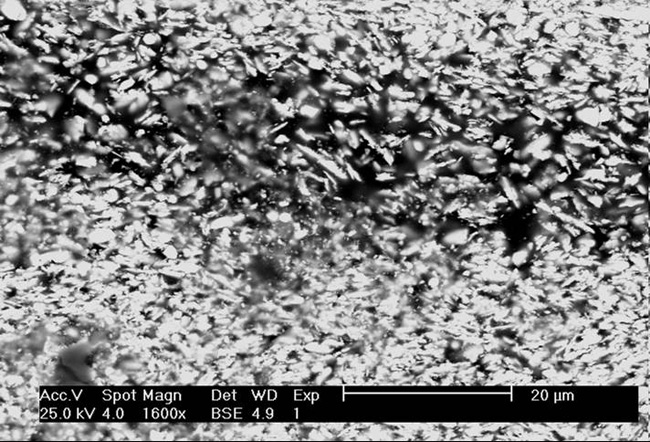
Fig. 13 Cross-section of modern lead white paint layer made with scanning electron micrograph (SEM).

Fig. 14 Cross-section of stack process lead white paint layer made with scanning electron micrograph (SEM).
The Old Dutch method and the quick process both result in lead white (basic lead carbonate), but with different particle sizes and shapes. The following two images are cross-sections of lead white paint; the first (fig. 13) is modern lead white pigment; the second (fig. 14) is a lead white pigment of the eighteenth century made by the stack process. The scanning electron microphotographs (SEM) show the particle shape and size of modern lead white pigment and the crystalline structure of stack process lead white.
The difference in the particle size and shapes of pigments in traditional and modern oil paints result in different properties, also known as “rheology.” To help us understand the differences, we should first define common terms used to describe paint rheology. Paint is a “pseudoplastic”; hence its behavior is similar to a liquid and a solid when force, such as stirring or brushing, is applied.
When the viscosity of a pseudoplastic substance decreases with increasing force, this is called “shearing thinning.” An example of a shear-thinning substance we are all familiar with is ketchup. If the viscosity increases with the increasing force, we call this “dilatant” or “shear thickening.” A commonly known dilatant substance is starch. When the viscosity of a substance decreases over time at a constant rate of force, this is called “thixotropy,” and the substance is said to be “thixotropic.”

Modern lead white does not exhibit much pseudoplastic flow.

Stack process lead white exhibits thixotropic pseudoplastic flow.
The particle size and shape of lead white pigment change the paint’s properties and behavior when brushed. This is clearly demonstrated in the video clip of two lead white oil paint samples. The paint on the right is modern lead white, while the pile of paint on the left is made with stack process lead white. When brushing thixotropic paint, it flows; when stopped, it holds its shape. The particle size and shape of traditional pigments often provide thixotropy.
Modern artists’ colors contain uniform and very fine pigment particles. On the other hand, traditional artists’ oil paint typically contains larger particle-sized pigments in various sizes and shapes. Although modern pigments have a variety of particle sizes and shapes, these are far simpler shapes and are smaller and more homogeneous than traditional pigments.
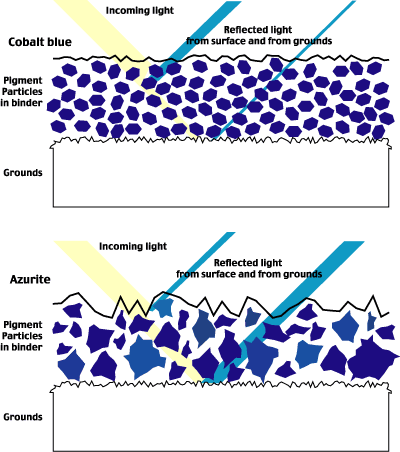
Fig. 15 Influence of the particle size and shape of pigments on the opacity, tinting strength, and hue of paint.
The particle size and shape influence the covering power of the paint, its tinting strength, and color. For example, modern paints’ small, uniform particles provide more coverage and higher tinting strength. The larger particle size and heterogeneous shapes of traditional pigments are typically more transparent, allowing more light to pass through the layers of paint to be reflected from the ground, thereby increasing its apparent luminosity. (See the bottom illustration of Fig. 15.)
Modern pigments’ small, uniform particles have greater tinting strength and purer color. Traditional pigments, however, offer the artist diverse chromatic diversity due to the heterogeneous size and shape of the particles. For example, crystalline hematite of about one micron size has a distinct violet tint differing from the bright red color of hematite with sub-micron particles.[4]
Advances in Paint Technology
Advances in technology not only changed the pigments used in oil paint but also changed how these paints were manufactured. In the nineteenth century, machinery was explicitly developed for colormen. James Rawlinson’s hand-operated, single-roll grinding mill was commended in 1804 by the Royal Society of Arts in London for the preparation of artists’ paint.[5] The development of powered mills made it possible for artists’ colormen to increase paint production, lowering the cost of paint while producing smoother pastes. The development of the collapsible tube in 1841 permitted colormen to supply oil paint in this convenient form to artists. It also allowed the paint to be stored for more extended periods.

Fig. 16 Rawlinson’s single-roll grinding mill for making artists’ colors.
By the twentieth century, pigment wetting, dispersion, and stabilization in paint binders were better understood, which lead to the use of additives that significantly changed the manufacture of paint. For example, aluminum stearate in artists’ oil paint in the early twentieth century has become a common additive in nearly all artists’ oil paint today.[6] While helping to prevent pigment and oil from separating, such additives altered the consistency of oil paints once familiar to artists before the twentieth century.
With increasing amounts of aluminum stearate, the oil pigment mixture becomes viscous, and by using an appropriate amount of aluminum stearate, the paint can gel using a lower pigment concentration. This dramatically mitigates or eliminates individual pigments’ effect on the consistency of paint.
Conclusion
Traditional paint made with traditional pigments results in the paint with chromatic diversity. Traditional pigments’ heterogeneous size and shape give novel, unique behavior to oil paint. Modern additives alter the behavior of paint, reducing or eliminating the individual effects created by pigments. Granular, crystalline pigments give a pleasing quality to paint films that cannot be had from fine, well-dispersed pigments produced for the modern paint industry.
This is the transcript of the lecture given by George O’Hanlon at the College Art Association 100th Annual Conference in Los Angeles on February 24, 2012.
Rublev Colours Artists Oils are Traditional Oil Paints
Why are Rublev Colours different from other modern commercial oil colors? One reason is that we use natural pigments or historical reproductions of pigments used by the old masters. Another reason is that we make Rublev Colours Artists’ Oils as they did before modern tube colors—without additives. Rublev Colours Artists Oils are formulated to maintain the unique characteristics of each pigment in oil. The character found in each tube of our oil colors is unique due to the pigment inside, giving the artist nearly limitless choices of texture, opacity, consistency, tone, and hue. With Rublev Colours, you experience the transparency of yellow ocher, the pale coolness of green earths, and the crystalline glitter of deep blue azurite.
The particles of natural pigments are at least six times the diameter of most synthetic pigments in modern artists’ colors. However, when we remember how predominantly crystalline or semi-crystalline pigments, such as azurite, malachite, lazurite, and so on, were used in old masters’ paintings, it is easy to understand how these beautiful surfaces with broken lights were obtained. An examination, for instance, of the surface of azurite blue under the microscope at once reveals the beautiful mass of blue and blue-green crystals, reflecting light in all directions and thus, of course, enhancing the decorative effect.
Pure Oil Color, No Additives
Rublev Colours Artists’ Oils do not contain additives, such as fillers, driers, and stabilizers—simply pigment and oil. We use refined linseed oil in our paint, sometimes with a small amount of heat-bodied linseed oil. Stabilizers, such as stearates and waxes, are not added that diminish the individual effects of pigments in oil. Therefore, you will find different consistencies from color to color due to the individual pigment characteristics and an occasional bit of free oil. Some colors brush out long, others short and buttery, and others are thixotropic.
Overall, Rublev Colours Artists’ Oils have longer brushing consistency than most tube colors available today, making them ideal for both bristle and soft-hair brushes in fine rendering, old master-like effects on both canvas and panels.
Where to Buy
See the range of
Notes
[1] Gettens, Rutherford John and Stout, George Leslie, Painting Materials: A Short Encyclopaedia, Dover Publications, 1966, p. 146.
[2] Kirby, Jo; Spring, Marika; Higgitt, Catherine, “The Technology of Eighteenth and Nineteenth Century Red Lake Pigment,” National Gallery Technical Bulletin, Vol. 28, p. 72.
[3] Patton, Temple C., Paint Flow and Pigment Dispersion, Interscience Publishers, 1964, p. 207.
[4] Hradila, David; Grygara, Tomáš; Hradilová, Janka; Bezdičkaa, Petr. “Clay and iron oxide pigments in the history of painting.” Applied Clay Science 22, 2003, p. 230.
[5] James Rawlinson, Transactions of the Society, Vol. xxii, 1804, p. 260–264. “Rawlinson’s Colour Grinding Mill,” Mechanic’s Magazine, Vol. 6, No. 152, Knight & Lacey, 1827, pp. 177–174.
[6] Tumosa, Charles S. “A Brief History of Aluminum Stearate as a Component of Paint,” WAAC Newsletter 23(3), 2001, pp. 10–11.












